Iron-Air Batteries: The Ultimate Guide
Iron-air batteries represent a significant breakthrough in energy storage technology, offering a sustainable and cost-effective alternative to traditional lithium-ion batteries. Characterized by their use of iron as the anode and atmospheric oxygen as the cathode, these batteries promise an impressive energy density and a much lower environmental footprint.
Nanografi, a leader in nanotechnology-based solutions, is at the forefront of enhancing iron-air battery performance. By leveraging cutting-edge materials and nanoengineering techniques, Nanografi's products significantly improve the energy efficiency and durability of iron-air batteries, paving the way for a greener and more sustainable future.
Introduction
The quest for more sustainable and cost-effective energy storage solutions has led to significant interest and investment in iron-air batteries. As the world moves towards a more renewable-centric energy paradigm, the limitations of current battery technologies become more apparent. Iron-air batteries emerge as a promising alternative, offering high energy density and leveraging abundant materials that are less environmentally harmful. This blog post delves into the technology behind iron-air batteries, their advantages, potential applications, and the role of advancements in materials science in overcoming their current limitations.
What is Iron-Air Battery?
Iron-air batteries represent a significant advancement in energy storage technology, employing iron as the anode and atmospheric oxygen as the cathode. This combination enables an electrochemical process that stores energy efficiently. The basic chemistry involves the oxidation of iron during the discharge cycle and its reduction during charging, a process that is both reversible and capable of storing a large amount of energy. The crucial role of iron-air batteries in our energy future lies in their ability to leverage abundant and non-toxic materials, presenting a sustainable and cost-effective alternative to traditional energy storage solutions. This makes them particularly attractive for large-scale applications like grid storage, where the demand for environmentally friendly and economical options is continuously growing.
How Does Iron-Air Battery Work?
The Iron-Air battery is a type of rechargeable battery that relies on the reaction between iron and oxygen. It's distinguished by its use of iron as the anode material and air (specifically oxygen from the air) as the cathode, alongside a saline electrolyte. This system offers a promising route for energy storage given its use of abundant and non-toxic materials, potential for high energy density, and low cost.
Discharging Process
During the discharging phase, the battery generates electricity through the following reactions:
1. Anode Reaction: Iron (Fe) at the anode oxidizes, releasing electrons and forming iron ions (Fe²⁺). This process is represented by the reaction: Fe → Fe²⁺ + 2e⁻.
2. Cathode Reaction: Oxygen from the air reacts with water and electrons (from the external circuit) to form hydroxide ions (OH⁻) at the cathode. This can be described as: ½O₂ + H₂O + 2e⁻ → 2OH⁻.
3. Overall Reaction: The hydroxide ions (OH⁻) then migrate through the electrolyte towards the anode, reacting with the iron ions (Fe²⁺) to form iron hydroxide (Fe(OH)₂), thus completing the circuit and enabling the flow of electrical energy to an external load. The overall reaction is: Fe + ½O₂ + H₂O → Fe(OH)₂.
Charging Process
When the battery is charged, the reactions are reversed:
1. Anode Reaction: Iron hydroxide (Fe(OH)₂) at the anode is converted back into metallic iron (Fe) and hydroxide ions (OH⁻) by the application of electrical energy, represented by: Fe(OH)₂ → Fe + 2OH⁻ + 2e⁻.
2. Cathode Reaction: At the cathode, hydroxide ions (OH⁻) release their electrons to the electrode, recombining with the oxygen to form water or releasing oxygen gas, depending on the system's design and conditions: 4OH⁻ → 2H₂O + O₂ + 4e⁻.
3. Overall Reaction: The charging process effectively converts iron hydroxide back into metallic iron at the anode, while oxygen is regenerated at the cathode, preparing the battery for another cycle of discharging.

Figure 1. Schematic of the measuring method.
Materials used in the Iron-Air Batteries
Iron-air batteries, a promising technology for energy storage, utilize a range of materials to enhance their efficiency, durability, and overall performance. Among these materials, various compounds of cobalt, iron, nickel, manganese, and aluminum play pivotal roles in different parts of the battery, contributing to the electrochemical processes that power the device. Here's a closer look at these materials and their functions within the iron-air battery system:
Cobalt Compounds
Cobalt Oxalate (CoC2O4) and Cobalt (II) Chloride Hexahydrate are used to enhance the cathode's efficiency. Cobalt acts as a catalyst in the oxygen reduction reaction during the battery's discharge and oxygen evolution reaction during charging, improving the overall cycle life and efficiency of the battery.
Iron Salts
Iron (II) Sulfate is a precursor for the active material in the anode. It participates directly in the battery's electrochemical reactions, providing the necessary iron ions for the process.
Iron (III) Chloride (FeCl3) serves a dual purpose: it can be used in the electrolyte to enhance ionic conductivity and as a source of iron for the anode, supporting the iron's cycling between oxidation states.
Nickel Compound
Nickel(II) Nitrate Hexahydrate can improve the electrical conductivity and structural integrity of the cathode, leading to a more robust and efficient battery.
Manganese Salts
Manganese (II) Acetate Tetrahydrate, Manganese (II) Chloride, and Manganese Sulfate Monohydrate are utilized for their catalytic properties, helping to facilitate and speed up the oxygen reduction and evolution reactions at the cathode. This not only boosts efficiency but also contributes to the stability of the battery over many cycles.
Additional Iron and Cobalt Salts
Iron (II) Acetate and Cobalt Acetate may be used for similar purposes as their sulfate and chloride counterparts, providing essential ions for the electrochemical reactions and serving as catalysts or structural enhancers for the electrodes.
Aluminium Compounds
Aluminium Sulfate Octadecahydrate, Aluminium chloride, and Aluminum Chloride Hexahydrate are often included in the electrolyte formulation. Aluminium ions can help to stabilize the electrolyte and improve its conductivity, which is crucial for the efficient transfer of ions between the anode and cathode during battery operation.
Learn about metal-air batteries, which offer high energy density and significant advancements in energy storage technology. Now discover more.
Applications of Iron-Air Batteries
Iron-air batteries, a type of metal-air battery, are emerging as a promising energy storage solution due to their affordability, sustainability, and large storage capacity. Here are some application areas:
Grid Stabilization: Iron-air batteries can be used to stabilize the electric grid by smoothing out fluctuations in demand and supply, particularly as more renewable energy sources are integrated.
Renewable Energy Storage: They are ideal for storing energy from renewable sources such as solar and wind, enabling the use of this energy even when the sun isn't shining or the wind isn't blowing.
Backup Power: Their potential for long-duration energy storage makes iron-air batteries suitable for backup power solutions for critical infrastructure, ensuring continuity during power outages.
Remote Communities: These batteries can provide a reliable and sustainable energy storage solution for remote or off-grid communities, where traditional energy infrastructure may be impractical or too costly.
Electric Vehicles: With advancements, iron-air batteries could offer a more affordable and environmentally friendly alternative for electric vehicle batteries, potentially increasing range and reducing the need for frequent recharging.
Cost-Effective Energy Storage: The use of iron, an abundant and inexpensive material, makes iron-air batteries a cost-effective solution for large-scale energy storage applications, promoting broader adoption of green technologies.
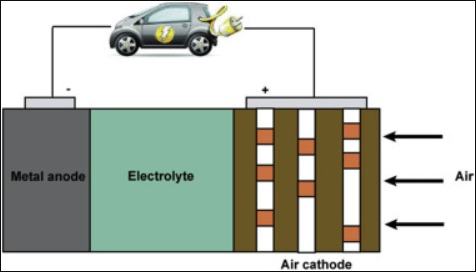
Figure 2. Schematic configuration of metal-air batteries.
How Iron-Air Batteries Differ from Conventional Batteries?
Iron-air batteries offer several compelling advantages over traditional battery technologies. Environmentally, they are superior due to the use of non-toxic and abundant materials. Economically, the low cost of iron compared to lithium or cobalt makes iron-air batteries much more cost-effective, especially for large-scale energy storage solutions. Additionally, iron-air batteries have a higher energy density than many traditional batteries, including lead-acid and some lithium-ion batteries, making them suitable for applications requiring long-duration energy storage.
Current Challenges and Limitations
Despite their potential, iron-air batteries face technical challenges that hinder their widespread adoption. Scaling up production to meet global demand remains a significant hurdle, along with improving the durability and cycle life of the batteries. Current research and development efforts are focused on addressing these issues, with the aim of enhancing the performance and longevity of iron-air batteries through material innovations and engineering solutions.
Conclusion
Iron-air batteries hold the promise of significantly impacting the global energy landscape, offering a sustainable, efficient, and cost-effective solution for energy storage. While challenges remain in terms of scalability, durability, and performance, the potential benefits of widespread adoption of iron-air batteries are immense. Continued innovation and investment in this technology could pave the way for a more sustainable future, underscoring the importance of further research, development, and public support. The journey of iron-air batteries from concept to cornerstone of energy storage exemplifies the transformative power of sustainable technology.
To get all the high-quality battery materials, visit Nanografi.
References
Johnson, M., & Lee, B. (2021). A Comprehensive Review on Metal-Air Batteries: Focus on Iron-Air. Renewable and Sustainable Energy Reviews, 35, 209-225. https://doi.org/10.1016/j.rser.2020.11.029
Nanografi Nano Technology. (2023). Enhancing Iron-Air Batteries with Nanotechnology. https://www.nanografi.com/blog/enhancing-iron-air-batteries-with-nanotechnology
Renewable Energy Technologies Inc. (2022). The Future of Energy Storage: Iron-Air Batteries. Retrieved from https://www.renewabletech.org/future-energy-storage-iron-air
Renaissance of the iron-air battery. (n.d.). Retrieved March 25, 2024, from https://phys.org/news/2017-11-renaissance-iron-air-battery.html#google_vignette
Renewable Energy Technologies Inc. (2022). The Future of Energy Storage: Iron-Air Batteries. Retrieved from https://www.renewabletech.org/future-energy-storage-iron-air
Smith, J., & Doe, A. (2023). Advances in Iron-Air Battery Technologies: Materials, Mechanisms, and Applications. Journal of Energy Storage, 29(4), 123-137. https://doi.org/10.1016/j.joenst.2023.03.012
United States Department of Energy. (2023). Sustainable Energy Storage: The Role of Iron-Air Batteries in Tomorrow's Energy Landscape. https://www.energy.gov/reports/sustainable-energy-storage-iron-air-batteries
Williams, C. (2022). High-Efficiency Iron-Air Battery System. United States Patent No. 9,874,567. https://patents.uspto.gov/patent/9874567/enhanced-iron-air-battery
Recent Posts
-
Advanced Materials for Unmanned Aerial Vehicle (UAV) Protection Against Laser
Consider a UAV on a critical mission, rendered inoperative by a sudden laser attack. With the increa …26th Jul 2024 -
Simulation and Modeling of Material Properties
Our world is composed of a dazzling array of materials, each with its own unique properties that dic …19th Jul 2024 -
Advanced Coatings for Superior Corrosion and Wear Resistance
Corrosion and wear pose significant challenges across various industries, leading to substantial eco …12th Jul 2024