Titanium Oxide Nanopowder/Nanoparticles Specifications
In recent times, there's been a significant push in the advancement of photodynamic therapy (PDT). This involves exploring new photosensitizing agents and better ways to deliver them.
One particularly promising method has been the combination of dyes and nanoparticles (NPs). This method not only boosts the selectivity of the photosensitizer but can also improve the overall effectiveness of the therapy. Among various substances used, Titanium Dioxide (Tio2) stands out. Its versatile properties make it useful in a range of applications, from purifying water to serving as a system for drug delivery. For cutting-edge applications like these, you can find the highest quality Titanium Dioxide nanoparticles and other nanomaterials in Nanografi's extensive product range.
Introduction
Particular attention should be paid to the last mentioned, but exceptional category containing metallic and metal oxide NPs, for example ZnO, Au, Fe2O3, TiO2. Several studies have indicated that the application of NPs in medicine can significantly improve the effectiveness of any existing therapies. Linking drugs with NPs can enhance their selective accumulation in diseased tissues as well as penetration abilities through cell membranes. Increasing the selectivity of drugs is a great challenge for modern medicine. This goal can be achieved by research focused on therapeutic systems with increased selectivity and reduced toxicity, accompanied by higher therapeutic efficiency.
What is Titanium Dioxide (TiO2)?
Titanium Dioxide (TiO2) is a naturally occurring white pigment sourced from minerals such as rutile and ilmenite. It is recognized for its bright white color, non-toxicity, stability, and high refractive index. These key properties make it a preferred choice in products like paints, sunscreens, cosmetics, and food coloring. Additionally, its capability to reflect ultraviolet (UV) light ensures it's a sought-after ingredient in sunscreens, providing protection against harmful UV rays.
Different forms of Titanium Dioxide
Titanium dioxide photocatalyst is a well-known and well researched photocatalyst due to its interesting properties which include stability, non-toxicity, biocompatibility, optical and electrical properties. It exists mainly in three different forms namely anatase, rutile and brookite and their structures are shown in Figure 1.
Anatase: This form possesses a tetragonal crystal structure. Anatase is frequently used in photovoltaic applications of TiO₂ due to its high photocatalytic activity. Even though it is less commonly found in nature compared to the rutile form, the anatase form is preferred in nanotechnology and certain industrial applications. Furthermore, the optical properties and surface reactivity of anatase make it intriguing for various scientific and technological applications.
Rutile: This form also has a tetragonal crystal structure and is the most common form of TiO₂ found in nature. Due to its optical properties, it is predominantly used in sunscreens and other cosmetic products. Rutile has a higher refractive index and a lower photocatalytic activity compared to anatase.
Brookite: This form is characterized by an orthorhombic crystal structure. However, brookite is less common than both anatase and rutile forms and is generally not employed in industrial and commercial applications. Nonetheless, it may be of interest for scientific research.
In addition, to learn more about rutile and anatase TiO2 nanoparticles and applications, you can visit our blog.
Figure 1. Different forms of TiO2.
The recent development of nanotechnology has proved that nanomaterials such as nano-sized titanium dioxide photocatalysts can have high activity in the photodegradation of a wide range of organic and inorganic contaminants in water. It is believed that photocatalysis will soon be recognized as one of the most effective means of dealing with various kinds of wastewater since organic pollutants can be completely degraded to harmless matter under normal conditions of temperature and pressure.
It is capable of degrading pollutants such as herbicides, carboxylic acids and alcohols completely to carbon dioxide, water and simple minerals. For it to be very effective, it should have certain properties such as suitable particle size, shape, crystallinity and a good ratio of anatase to rutile. This is the reason why most researchers have been trying to use different methods to get particles with suitable characteristics for environmental remediation or for other applications of interest. Several studies have proved that TiO2 nanostructures, in particular, titanium dioxide nanotubes (TNTs) have their performance improved in photovoltaics and photocatalysis compared to nanoparticulate forms of titanium oxide. Recently, bundles and arrays of TNTs with different qualities have been synthesized by a variety of different techniques such as template-assisted, sol-gel, hydrothermal, electro-anodization, chemical vapor deposition and physical vapor deposition.
There are other various techniques for preparing titanium dioxide nanoparticles and these include reverse micelles, the sol-gel process, the metal organic chemical vapor deposition (MOCVD), gas phase (aerosol) synthesis, wet-chemical synthesis by precipitation of hydroxides from salts, microemulsion-mediated methods and electrochemical synthesis. These methods can be divided into five general groups namely sol-gel, deposition methods, sonochemical and microwave-assisted methods, hydro/solvothermal methods and oxidation methods.
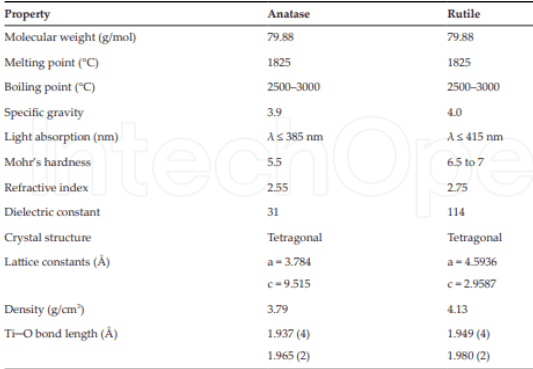
Table 1: It shows some of the structural and physical properties of the anatase and rutile phase of titanium dioxide.
Synthetic Methods of Titanium Dioxide
Deposition Methods
In these methods, materials in the vapor state are condensed to form a solid phase material. The process is normally carried out in a vacuum chamber and if a chemical reaction takes place, it is called chemical vapor deposition (CVD) and physical vapor deposition (PVD) if no reaction occurs. Examples of CVD include electrostatic spray hydrolysis, diffusion flame pyrolysis, thermal plasma pyrolysis, ultrasonic spray pyrolysis, laser-induced pyrolysis and ultrasonic-assisted hydrolysis. TiO2 films with grain size less than 30 nm and TiO2 nanoparticles with sizes less than 10 nm were synthesized by pyrolysis of titanium tetraisopropoxide (TTIP) in a helium/oxygen atmosphere. Thermal plasma synthesis and spray pyrolysis have been used in some studies but they are complex, capital and energy-intensive and the properties of the powder are not easy to control.
- Electrophoretic deposition
- Spray pyrolysis
Oxidation Methods
These methods involve the oxidation of titanium metal using oxidants or anodization. Anodization of titanium sheet under a voltage betwen 10 and 20 V in 0.5% hydrogen fluoride leads to the formation of aligned TiO2 nanotubes whose diameter is controlled by varying the applied voltage.
Sonochemical and Micro-Wave Assisted Methods
The sonochemical method has been applied to produce highly photoactive TiO2 nanoparticles by the hydrolysis of titanium tetraisopropoxide (TTIP) in pure water or in an ethanol/water mixture under ultrasonic radiation. Sonochemistry arises from acoustic cavitation which is the formation, growth and collapse of bubbles within a liquid medium. Heat (~5000 K) and high pressures (~1000 atm) are produced by cavitational collapse.
In microwave-assisted methods, there is a use of microwaves which are electromagnetic waves with frequencies which range from 0.3 to 300 GHz and with wavelengths between 1 mm and 1 m. According to Zhu and Chen [36], microwave heating involves two main mechanisms namely dipolar polarization and ionic conduction. Any materials that contain mobile electric charges such as polar molecules or conducting ions are generally heat by microwaves.
Sol-Gel Methods
The sol-gel process produces fine, spherical powders of uniform size and has been widely used to synthesize TiO2 materials and normally proceeds via an acid-catalyzed step of titanium (IV) alkoxides. One of the most attractive features of the sol-gel process is the possibility to shape the resulting material into desired forms such as fiber, film and monodispersed powder.
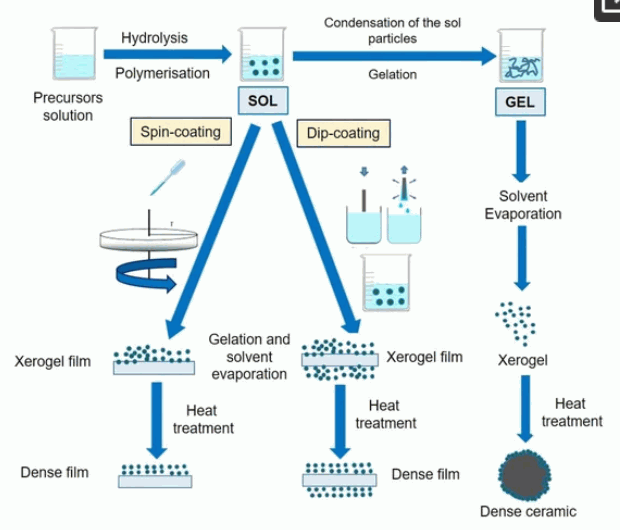
Figure 2: Schematic representation of the typical sol-gel route.
Template-Based Methods
Template-assisted synthesis is an easy, cost effective and highly versatile approach to fabricate nanostructures. Whenever microporous or nanoporous materials are utilized as templates, one-dimensional (1D) nanostructures can be integrated by saving a material of decision inside the format’s nano-channels. Michailowski et al. showed the synthesis of TiO2 nanotubes via a thermal decomposition process of Ti(Oi─Pr)4 using anodic aluminum oxide as a template [77]. Correspondingly, Liang et al. revealed the synthesis of TiO2 nanotubes through atomic layer deposition [78]. Anodic aluminum oxide was utilized as the template, and TiCl4 was used as the precursor for the atomic layer deposition of TiO2.
Applications of Titanium DiOxide (TiO2) Nanoparticles
Titanium dioxide (TiO2) has a wide range of applications in medicine and related fields, beyond just drug delivery systems. Here is a summary of its various applications:
Dyes
Titanium dioxide is the most commonly used white pigment in dyes. Thanks to its high opacity capacity, it makes the dyes more covering. It can be used in both water-based and solvent-based dyes.
Cosmetic
-Titanium dioxide (TiO₂) is an inorganic compound extensively employed in the cosmetics industry. It is particularly favored in sun protection formulations due to its ability to block ultraviolet (UV) radiation.
-TiO₂'s high refractive index imparts a robust reflective and scattering capacity against UV rays, facilitating the formation of an effective protective layer on the skin. Additionally, it is utilized as a white pigment in makeup products, especially in foundations and powders. However, due to research on the potential health effects of nanoparticles, caution must be exercised regarding the size and formulation of TiO₂ used in cosmetics.
Dentistry
-Titanium dioxide is used in toothpaste and teeth whitening products due to its photochemical activity, which helps improve tooth personal care and teeth whitening.
-It is applied in the treatment of tooth hypersensitivity, which is characterized by sharp pain from exposed dentine in response to stimuli.
Pharmaceutical Sciences
-Titanium dioxide is used as a pharmaceutical excipient in tablet manufacturing.
-TiO2 nanoparticles can act as catalytic systems to eliminate hazardous chemical and pharmaceutical pollutants.
-They can be used to mask the bitter taste of drugs, improving the overall taste of medicines.
Wastewater Treatment
-TiO2 nanoparticles are cost-effective and resistant to corrosion, making them suitable for wastewater treatment.
-They can be used for the remediation of surface water by preconcentrating and extracting heavy metals from water.
Graphene is also utilized in water filtration, for a detailed explanation, visit our academic blog post.
-TiO2 nanoparticles can function as nanocatalysts in the degradation of pollutants, such as the degradation of dyes like methyl orange.
Researchers have used TiO2-impregnated titanium mesh filters to remove indoor pollutants, including cigarette smoke.
Food Industry
-Titanium dioxide is used in food products like white-colored sauces, confectioneries, nondairy creamers, and cheese.
-In foods, it restores whiteness to creamy products and acts as a barrier for colors in confectionery products.
Graphene, on the other hand, plays a significant role in packaging, food safety, and hygiene in the food industry. To learn more, read our blog post.
Photodynamic Therapy
-TiO2 nanoparticles possess excellent photochemical properties and high biocompatibility, making them suitable for photodynamic therapy.
-They can produce reactive oxygen species when exposed to light, leading to cell death in nearby tissues.
-Modifications like doping and functionalization can shift their absorption bands for better therapeutic applications.
-Strategies like PEG chain functionalization can mitigate agglomeration in physiological pH, ensuring consistent dosing and clinical effects.
It's worth noting that while TiO2 nanoparticles show promise in various applications, their behavior and potential toxicity in the body require further investigation to ensure safe and effective use in medical and therapeutic contexts.
Why is titanium dioxide used in sunscreen, and how does it provide UV protection? To find the answer, read the blog.
Conclusion
In conclusion, Titanium Dioxide (TiO2) nanoparticles offer immense potential in fields like photodynamic therapy and water purification. While they are widely accessible and cost-effective, fully harnessing their potential in medical applications presents challenges. Addressing drawbacks such as the need for UV light activation is crucial. By integrating strategies like combining TiO2 with up-conversion nanoparticles and employing surfactants, we can optimize their use. Nevertheless, understanding TiO2's behavior and potential toxicity remains essential as we delve deeper into its medical applications.
To support your research and projects, Nanografi introduces high-quality titanium dioxide products to you.
References
Analyzed: Anatase Titanium Dioxide Nanoparticles - Nanografi Nano Technology. (n.d.). Retrieved February 7, 2024, from https://nanografi.com/blog/analyzed-anatase-titanium-dioxide-nanoparticles-descriptions-usage-and-applications/
Applications of Graphene in Food and Beverage Packaging - Nanografi Nano Technology. (n.d.). Retrieved February 7, 2024, from https://nanografi.com/blografi/applications-of-graphene-in-food-and-beverage-packaging/
Banerjee, K., & Thiagarajan, P. (2015). A Review of Titanium Di Oxide Nanoparticles - Synthesis, Applications and Toxicity Concerns. Nanoscience &Nanotechnology-Asia, 4(2), 132–143. doi: 10.2174/2210681204666141117234425
De Matteis, V., Cannavale, A., & Ayr, U. (2020). Titanium Dioxide in Chromogenic Devices: Synthesis, Toxicological Issues, and Fabrication Methods. Applied Sciences 2020, Vol. 10, Page 8896, 10(24), 8896. https://doi.org/10.3390/APP10248896
Different forms titanium dioxide [1]. | Download Scientific Diagram. (n.d.). Retrieved February 7, 2024, from https://www.researchgate.net/figure/Different-for...
Graphene Water Filtration - Nanografi Nano Technology. (n.d.). Retrieved February 7, 2024, from https://nanografi.com/blog/graphene-water-filtration/
Hollingsworth, L. R., Conduff, J. H., Balzer, C. J., Tran, S. K., Hamid, W., & Rakes, L. E. (2015). Synthesis and Toxicity of Lipid-Coated-Titanium Oxide Nanoparticles. Journal of Undergraduate Materials Research, 5(0). doi: 10.21061/jumr.v5i0.1533
Nyamukamba, P., Okoh, O., Mungondori, H., Taziwa, R., & Zinya, S. (2018). Synthetic Methods for Titanium Dioxide Nanoparticles: A Review. Titanium Dioxide - Material for a Sustainable Environment. doi: 10.5772/intechopen.75425
Peng, T., Zhao, D., Dai, K., Shi, W., & Hirao, K. (2005). Synthesis of Titanium Dioxide Nanoparticles with Mesoporous Anatase Wall and High Photocatalytic Activity. The Journal of Physical Chemistry B, 109(11), 4947–4952. doi: 10.1021/jp044771r
Titanium Dioxide Reshaping the Field of the Cosmetic Industry - Nanografi - Nanografi Nano Technology. (n.d.). Retrieved February 7, 2024, from https://nanografi.com/blog/titanium-dioxide-reshaping-the-field-of-the-cosmetic-industry-nanografi-/
Waghmode, M. S., Gunjal, A. B., Mulla, J. A., Patil, N. N., & Nawani, N. N. (2019). Studies on the titanium dioxide nanoparticles: biosynthesis, applications and remediation. SN Applied Sciences, 1(4). doi: 10.1007/s42452-019-0337-3
Ziental, D., Czarczynska-Goslinska, B., Mlynarczyk, D. T., Glowacka-Sobotta, A., Stanisz, B., Goslinski, T., & Sobotta, L. (2020). Titanium Dioxide Nanoparticles – Prospects and Applications in Medicine. doi: 10.20944/preprints202002.0271.v1
Recent Posts
-
Advanced Materials for Unmanned Aerial Vehicle (UAV) Protection Against Laser
Consider a UAV on a critical mission, rendered inoperative by a sudden laser attack. With the increa …26th Jul 2024 -
Simulation and Modeling of Material Properties
Our world is composed of a dazzling array of materials, each with its own unique properties that dic …19th Jul 2024 -
Advanced Coatings for Superior Corrosion and Wear Resistance
Corrosion and wear pose significant challenges across various industries, leading to substantial eco …12th Jul 2024






