Carbides: From Atomic Structure to Industrial Applications - Nanografi
Carbides are carbon-based compounds including metals. They're known for their hardness, brittleness, and high melting points. They form through reactions like carbon directly reacting with metals or by heating metals with carbon sources.
These qualities make them valuable in various industries, from cutting tools to coatings and abrasives. Furthermore, the article delves into potential future research and applications, hinting at the pivotal role carbides might play in emerging fields like aerospace and energy storage. The versatile nature of carbides, addressing industrial needs and fostering innovation, is emphasized, with ongoing research striving to augment their properties and reveal untapped potentials. At Nanografi, we offer superior performance through carbides' unique durability and versatility. Our advanced carbide solutions are ready to meet industry needs and support innovation.
Introduction
The most common and well-known carbide is calcium carbide (CaC2), which is produced by heating lime and carbon together. It is widely used in the production of acetylene gas, which has applications in welding and as a chemical feedstock. Tungsten carbide (WC) is another prominent carbide that is extremely hard and is used in cutting tools, drills, and abrasives. Cemented carbides, which are composed of tungsten carbide particles embedded in a metallic binder, are used in high-performance cutting tools due to their excellent hardness and wear resistance. Other examples of carbides include silicon carbide (SiC), boron carbide (B4C), titanium carbide (TiC), and many more. These carbides find applications in various industries, including metallurgy, ceramics, electronics, and manufacturing.
Types and Classification of Carbides
Carbides can be classified into different types based on their composition, structure, and properties. Here are some common types of carbides
1. Binary Carbides: These carbides consist of carbon and a single metallic element. Examples include calcium carbide (CaC2), silicon carbide (SiC), and boron carbide (B4C).
2. Interstitial Carbides: These carbides are formed when carbon atoms occupy the interstitial sites within a metallic lattice. They are typically very hard and have high melting points. Tungsten carbide (WC) and Titanium carbide (TiC) are examples of interstitial carbides.
3. Cemented Carbides: Also known as hardmetal or tungsten carbide-cobalt (WC-Co), cemented carbides are composite materials composed of tungsten carbide particles bonded together by a metallic binder, usually cobalt. They are widely used in cutting tools, mining tools, and wear-resistant components.
4. Transition Metal Carbides: These carbides are formed by transition metals, such as titanium carbide (TiC), vanadium carbide (VC), and niobium carbide (NbC). They possess excellent hardness, high melting points, and good resistance to wear and corrosion.
5. Salt-Like Carbides: These carbides are characterized by a crystal structure similar to that of ionic compounds. Calcium carbide (CaC2) is a common example of a salt-like carbide.
6. Covalent Carbides: These carbides are formed by covalent bonding between carbon and a metal or semi-metal element. Silicon carbide (SiC) is the most widely used covalent carbide. It has excellent mechanical properties, high thermal conductivity, and resistance to chemical reactions.
7. Reactive Carbides: These carbides are highly reactive and can undergo chemical reactions with other elements or compounds. Calcium carbide (CaC2) is a reactive carbide that produces acetylene gas when exposed to water.
These are just a few examples of the different types of carbides. Each type has unique properties and applications, making them valuable in various industries and fields of research.
The Structure and Composition of Carbides
Carbides are compounds composed of carbon and one or more metallic elements. They can be classified into various types based on their composition, structure, and properties. Some common types of carbides include binary carbides (e.g., CaC2, SiC, B4C), interstitial carbides (e.g., WC, TiC), cemented carbides (e.g., WC-Co), transition metal carbides (e.g., TiC, VC, NbC), covalent carbides (e.g., SiC), and reactive carbides. Binary carbides consist of carbon and a single metallic element, while interstitial carbides form when carbon atoms occupy the interstitial sites within a metallic lattice. Cemented carbides are composite materials with hard carbide particles bonded by a metallic binder, while transition metal carbides are formed by transition metals and can exhibit different crystal structures. Covalent carbides result from covalent bonding between carbon and a metal or semi-metal element. The composition and structure of carbides influence their properties, such as hardness, melting point, thermal conductivity, and resistance to wear and corrosion. Carbides find applications in various industries, including metallurgy, ceramics, electronics, and manufacturing, due to their unique properties and versatility.
Atomic and Molecular Structure
Carbides exhibit different atomic and molecular structures depending on their specific type. Binary carbides, such as calcium carbide (CaC2), silicon carbide (SiC), and boron carbide (B4C), consist of carbon and a single metallic element. They have crystal structures that can vary, with different arrangements of atoms and bonding patterns. Interstitial carbides, such as tungsten carbide (WC) and titanium carbide (TiC), form when carbon atoms occupy the interstitial sites within a metallic lattice. Tungsten carbide has a close-packed lattice structure with carbon atoms in the interstitial sites, while titanium carbide has a face-centered cubic lattice structure with carbon atoms in the interstitial sites. Cemented carbides, like tungsten carbide-cobalt (WC-Co), are composite materials where hard carbide particles are bonded together by a metallic binder. The hard carbide particles have their specific crystal structures, while the metallic binder, usually cobalt, fills the spaces between the carbide particles, forming a metallic matrix.
Understanding the atomic and molecular structures of carbides is important for comprehending their properties and behavior. The arrangement of atoms and bonding patterns directly affect characteristics such as hardness, thermal conductivity, and reactivity, which are vital for their applications in various industries.
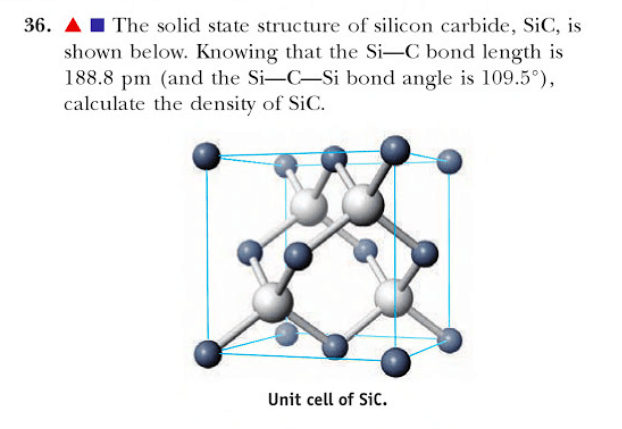
Figure 1. Solid state structure of silicon carbide.
Diversity of Compositions
Carbides exhibit a diverse range of compositions, which contribute to their unique properties and applications. Binary carbides, such as calcium carbide (CaC2), silicon carbide (SiC), and boron carbide (B4C), consist of carbon and a single metallic element. Interstitial carbides, like tungsten carbide (WC) and titanium carbide (TiC), form when carbon atoms occupy the interstitial sites within a metallic lattice. Cemented carbides, such as tungsten carbide-cobalt (WC-Co), are composite materials composed of hard carbide particles bonded together by a metallic binder.
Transition metal carbides, like titanium carbide (TiC) and niobium carbide (NbC), and covalent carbides, such as silicon carbide (SiC) and boron carbide (B4C), showcase further diversity in compositions. Each type of carbide possesses unique properties, including hardness, thermal conductivity, and chemical reactivity, which make them suitable for various industries and applications. Understanding the diversity of compositions in carbides is crucial for selecting the appropriate carbide material for specific applications, considering factors such as desired properties, environmental conditions, and manufacturing processes.
Characteristics of Carbides
Carbides possess several key characteristics that make them valuable materials in various industries and applications. They are known for their exceptional hardness, often rivaling that of diamond, which makes them highly resistant to wear and abrasion. Carbides also have high melting points, allowing them to withstand high temperatures without significant degradation.
Another important characteristic of carbides is their thermal conductivity, which enables efficient heat dissipation. Many carbides are chemically inert, making them resistant to chemical reactions and corrosion. This property is particularly useful in harsh and corrosive environments.
Carbides exhibit excellent wear resistance, making them suitable for applications subjected to high levels of friction. Some carbides, like silicon carbide (SiC), also possess semiconducting properties, making them valuable in the electronics industry. To learn detailed information about silicon carbide, read our blog.
Additionally, carbides offer versatility, as their properties can be tailored by adjusting their composition, structure, and processing techniques. This adaptability allows for a wide range of applications in industries such as aerospace, automotive, mining, construction, and electronics.
Overall, the hardness, high melting points, thermal conductivity, chemical inertness, wear resistance, electrical conductivity, and versatility of carbides contribute to their importance and utility across various fields and applications.
High Melting Points
Carbides have exceptionally high melting points, exceeding those of pure metals. This attribute arises from strong carbon-metal bonding, carbide crystal structures, and interstitial carbon. These high melting points confer resistance to degradation under extreme heat, finding use in manufacturing, aerospace, and energy sectors. Applications range from furnace parts to rocket nozzles and cutting tools, vital for durability and performance in high-temperature conditions. Carbides' versatility in various industries owes much to their capacity to retain integrity and hardness at elevated temperatures.
Durability and Strength
Carbides are valued for their durability and strength. They are exceptionally hard, wear-resistant, and maintain their shape and properties under demanding conditions. Carbides exhibit strength at high temperatures and possess high compressive strength. These qualities make carbides suitable for a wide range of applications in various industries.
Carbides: Properties and Applications
Carbides are compounds consisting of carbon and one or more metallic elements. They possess properties such as exceptional hardness, high melting points, thermal stability, and corrosion resistance. These properties make carbides suitable for a wide range of applications.
Carbides are commonly used in cutting tools, such as drills and milling cutters, due to their hardness and wear resistance. They are also employed in grinding wheels and abrasive papers for grinding and polishing operations. Carbides find applications in high-temperature environments, such as furnace components and rocket nozzles, thanks to their high melting points and thermal stability. Certain carbides exhibit good electrical conductivity and are used in the production of acetylene gas for welding and lighting. Carbides with excellent corrosion resistance, like boron carbide, are used in armor plating and abrasive materials. Cemented carbides, which are composites of carbide particles in a metallic binder, are widely used in cutting tools, wear-resistant components, and mining tools. Carbides are also utilized as coatings to enhance the hardness, wear resistance, and corrosion resistance of metal surfaces. In the semiconductor industry, silicon carbide is employed in power electronic devices and LED lighting due to its unique properties. Carbides have even found applications in the nuclear industry as fuel materials in certain types of reactors.
Overall, carbides are versatile materials with a wide range of applications in industries such as manufacturing, engineering, electronics, and energy. They provide desirable properties that include hardness, thermal stability, corrosion resistance, and electrical conductivity.
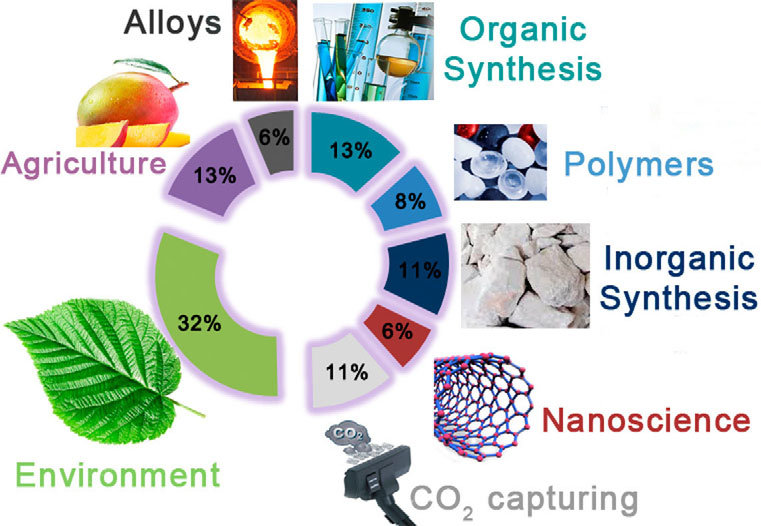
Figure 2. Main areas for the applications of calcium carbide.
Silicon Carbide (SiC):
Silicon carbide (SiC) is a compound known for its exceptional hardness, high melting point, thermal conductivity, and chemical inertness. It finds various applications across different industries. Silicon carbide is widely used as an abrasive material in grinding wheels, sandpapers, and abrasive papers. It is also employed in the production of refractory materials that can withstand extreme temperatures, such as kiln furniture and high-temperature furnace components. In the field of power electronics, silicon carbide is utilized for high-voltage devices and power semiconductors due to its high breakdown voltage, ability to handle high temperatures, and improved efficiency compared to traditional silicon-based devices. It is also used in the semiconductor industry for devices like diodes, transistors, and RF devices. In the automotive industry, silicon carbide is gaining popularity for electric vehicle power electronics and charging infrastructure due to its high-temperature capabilities and efficiency.
Silicon carbide plays a significant role in LED lighting, enabling the production of high-efficiency LEDs with improved performance and durability. It is employed in the renewable energy sector for applications such as solar inverters and wind turbine systems, contributing to high-power conversion and energy management. Overall, silicon carbide is a versatile material with a wide range of applications in abrasives, refractory materials, power electronics, LED lighting, semiconductors, automotive industry, and renewable energy systems. Its exceptional properties make it valuable in various industries where hardness, high-temperature tolerance, thermal conductivity, and chemical inertness are essential.
Tungsten Carbide (WC):
Tungsten carbide (WC) is a compound known for its exceptional hardness, high melting point, wear resistance, and chemical inertness. It finds numerous applications in various industries. Tungsten carbide is widely used in the manufacturing of cutting tools such as drills, end mills, and inserts, due to its hardness and wear resistance. It is also extensively employed in mining and drilling applications, where it can withstand the demanding conditions of hard rock drilling and excavation. The material is used in wear parts and tooling applications, including wear-resistant nozzles, valves, bearings, and components for pumps and machinery subjected to abrasive environments. In metal forming and stamping, tungsten carbide is utilized in dies and punches for shaping and forming metals under high pressure. Tungsten carbide has found its way into the jewelry industry due to its hardness and scratch resistance, making it a popular choice for wedding bands, rings, and bracelets. It is also used in ballistic armor and armor-piercing projectiles because of its high density and hardness, offering effective protection against ballistic threats. In addition to industrial applications, tungsten carbide is used in rotary and cutting tools for engraving, milling, and woodworking. It is even employed in sports equipment such as darts, golf clubs, and fishing weights, providing improved stability and control due to its high density. Overall, tungsten carbide is a versatile material with exceptional properties, making it suitable for applications that require hardness, wear resistance, and durability. Its uses span across cutting tools, mining and drilling, wear parts, metal forming, jewelry, ballistic armor, rotary tools, and sports equipment.
Carbide burr
Carbide burrs are cutting tools used in milling, shaping, grinding, and deburring operations. They are made from tungsten carbide, a hard and durable material known for its exceptional hardness and wear resistance. Tungsten carbide is brazed or welded onto a shank to create the cutting edges of the burr. Carbide burrs are commonly used in milling applications, particularly in metalworking and machining. They are attached to rotary tools and used to remove material, shape contours, and create precise cuts. The sharp cutting edges and hardness of the tungsten carbide allow for efficient and precise milling operations on various materials. Carbide burrs come in different shapes and sizes, such as cylindrical, ball-shaped, cone-shaped, and flame-shaped, to accommodate different milling requirements. They find applications in industries like automotive, aerospace, metal fabrication, woodworking, and jewelry making.
In summary, carbide burrs, made from tungsten carbide, are versatile cutting tools used in milling operations. They leverage the hardness and wear resistance of tungsten carbide to provide efficient material removal, shaping, and cutting capabilities in a wide range of applications.
Titanium Carbide (TiC):
Titanium carbide (TiC) is a compound known for its exceptional hardness, high melting point, and chemical inertness. It is widely used in various applications. It is a versatile material used in hard coatings, cutting tools, wear-resistant components, refractory materials, additive manufacturing, and electronic components. Its hardness, high melting point, and chemical inertness make it valuable in industries where durability, wear resistance, and high-temperature performance are required.
To get more information about Titanium carbide, read our blog.
Boron Carbide (B4C):
Boron carbide (B4C) is a compound known for its exceptional hardness, high melting point, and lightweight nature. Boron carbide is a versatile material used in armor and ballistic protection, abrasives, nuclear applications, nozzle production, ceramic and refractory materials, and cutting tools. Its exceptional hardness, high melting point, lightweight nature, and chemical inertness make it valuable in industries requiring hardness, wear resistance, lightweight design, and high-temperature performance.
Tantalum Carbide (TaC):
Tantalum carbide is a valuable material with high melting point, exceptional hardness, chemical stability, and moderate electrical conductivity. It finds applications in cutting tools, refractory materials, wear-resistant components, cutting tool coatings, electronic components, and additive manufacturing. Its properties make it suitable for industries requiring high-temperature performance, wear resistance, hardness, and electrical conductivity.
Niobium Carbide (NbC):
Niobium carbide is a valuable material with high melting point, exceptional hardness, chemical stability, and thermal conductivity. It finds applications in cutting tools, wear-resistant components, refractory materials, additive manufacturing, coatings, and nuclear applications. Its properties make it suitable for industries requiring high-temperature performance, wear resistance, and hardness.
Vanadium Carbide (VC):
Vanadium carbide is also a valuable material with high melting point, exceptional hardness, chemical stability, and thermal conductivity. It finds applications in cutting tools, wear-resistant components, refractory materials, additive manufacturing, coatings, and cemented carbides. Its properties make it suitable for industries requiring high-temperature performance, wear resistance, and hardness.
Zirconium Carbide (ZrC):
Zirconium carbide has a high melting point, exceptional hardness, chemical stability, thermal conductivity, and refractory properties. It finds applications in refractory materials, cutting tools, coatings, ceramic materials, nuclear applications, and abrasives. Its properties make it suitable for industries requiring high-temperature performance, wear resistance, hardness, and corrosion resistance.
Hafnium Carbide (HfC)
Hafnium carbide also have a high melting point, exceptional hardness, chemical stability, thermal conductivity, and refractory properties. It finds applications in refractory materials, cutting tools, coatings, ceramic materials, nuclear applications, and aerospace applications. Its properties make it suitable for industries requiring high-temperature performance, wear resistance, hardness, and corrosion resistance.
The Role of Carbides in Various Industries
Carbides, such as tungsten carbide and silicon carbide, play important roles in various industries. They are used in cutting tools for metalworking, drilling and excavation in mining and construction, components in the oil and gas industry, critical parts in aerospace and defense systems, automotive applications, semiconductor manufacturing, and refractory materials for high-temperature environments. Carbides provide exceptional hardness, wear resistance, high melting points, and thermal stability, making them valuable materials in industrial applications.
Use in the Tool and Die Industry
Carbides are widely used in the tool and die industry for their exceptional hardness, wear resistance, and toughness. They are employed in cutting tools, forming and stamping dies, wire and thread dies, punches and blanking dies, injection mold components, and die casting tools. Carbides improve tool life, cutting speeds, and productivity, enhancing efficiency and accuracy in metalworking and manufacturing processes.
Importance in the Steel Industry
Carbides are crucial in the steel industry for their wear resistance, cutting capabilities, tooling and die applications, blast furnace linings, coatings, and alloying properties. They improve efficiency, productivity, and the quality of steel manufacturing processes, resulting in high-performance and durable steel products.
Applications in the Electronics Sector
Carbides have important applications in the electronics sector, including semiconductors (such as silicon carbide), LEDs and lighting, MEMS devices, power electronics, thermal management, and RF/microwave components. Carbides offer properties like high thermal conductivity, high-temperature stability, and wide bandgap, making them valuable for advanced electronic devices and systems.
Other Industrial Applications
Carbides have diverse industrial applications, including chemical synthesis, mining, metal casting, welding, automotive, medical, and abrasives. They provide properties such as hardness, wear resistance, and corrosion resistance, making them valuable materials in various industries.
Future of Carbides
The future of carbides is promising, with advancements in materials, manufacturing processes, and applications. Researchers are developing advanced carbide materials with improved properties, exploring sustainable manufacturing methods, and leveraging additive manufacturing for customized components. Carbides are finding applications in emerging fields like energy, quantum computing, and microfabrication. Coatings and surface engineering using carbides are also gaining traction. The future of carbides involves continuous innovation and their role in advancing technology and meeting industry needs.
Emerging Research and Developments
Carbides are undergoing emerging research and developments in areas such as nanostructured carbides, carbide-based composites, sustainable production methods, carbide-based sensors, energy applications, and carbide coatings for extreme environments. These advancements aim to enhance properties, expand capabilities, and address industry needs.
Potential New Applications and Uses
Carbides have the potential for new applications in aerospace, energy storage, biomedical, high-performance ceramics, electronics cooling, additive manufacturing, and environmental sectors. Ongoing research and advancements are uncovering opportunities for utilizing carbides in various industries.
Conclusion
In conclusion, the chemical structure, physical properties, and industrial applications of carbides are highly diverse. With features such as high hardness, thermal stability, and resistance to high temperatures, they play a significant role in various sectors. Moreover, with ongoing research and innovations, it is anticipated that the potential of carbides will be further expanded, enabling their utilization in new fields. To advance future technology and explore new potential realms for carbides, Nanaografi is committed to daily efforts, bringing forth products tailored to this purpose.
We invite you to explore our products offerings by visiting our website.
References
Carbide Burr tips and information. (n.d.). Retrieved March 14, 2024, from https://www.carbideanddiamondtooling.com/Carbide-Burrs_b_3.html
Cemented carbide - Wikipedia. (n.d.). Retrieved March 14, 2024, from https://en.wikipedia.org/wiki/Cemented_carbide
Katoh, Y., & Snead, L. L. (2019). Silicon carbide and its composites for nuclear applications – Historical overview. Journal of Nuclear Materials, 526, 151849. https://doi.org/10.1016/J.JNUCMAT.2019.151849
Main areas for the application of calcium carbide. The schematic... | Download Scientific Diagram. (n.d.). Retrieved March 14, 2024, from https://www.researchgate.net/figure/Main-areas-for-the-application-of-calcium-carbide-The-schematic-representation-provides_fig3_332145935
Silicon Carbide (SiC) Micron and Nano Powder - Nanografi Nano Technology. (n.d.). Retrieved March 14, 2024, from https://nanografi.com/blog/silicon-carbide-sic-micron-and-nano-powder/
Titanium Carbide Nanoparticles: History, Properties, Synthesis, Applications - Nanografi Nano Technology. (n.d.). Retrieved March 14, 2024, from https://nanografi.com/blog/titanium-carbide-nanoparticles-history-properties-synthesis-applications/
What is SiC structure? - Compound Semiconductor News. (n.d.). Retrieved March 14, 2024, from https://www.csfusion.org/faq/what-is-sic-structure/
Recent Posts
-
Reducing the Carbon Footprint of Nanomaterials
The production of nanomaterials is vital for numerous advanced applications, from healthcare to elec …26th Apr 2024 -
Nanocomposites in Food Packaging
The utilization of nanocomposites in food packaging represents a significant advancement in the fiel …19th Apr 2024 -
What is the Difference Between 7075 and 6061 Aluminum Alloy?
When comparing 7075 aluminum alloy to 6061 aluminum alloy, it's essential to understand their disti …5th Apr 2024







