NMC and Lithium Batteries: A Groundbreaking Relationship in Energy Storage - Nanografi
The relationship between Lithium Nickel Manganese Cobalt Oxide (NMC) and lithium batteries is revolutionary in the field of energy storage. NMC stands out as a vital component of lithium-ion batteries.
Comprising nickel, manganese, and cobalt, NMC composition is known for its high energy density. NMC batteries excel in longevity, cost-efficiency, and high performance. Moreover, NMC batteries find widespread use in applications like electric vehicles and solar energy storage systems. It's evident that NMC has a significant role in the future of lithium-ion batteries, as these components make energy storage technologies safer, more efficient, and more sustainable. Nanografi, a leading supplier and solution partner specializing in high-quality nanomaterials and nanotechnology-based products, we offer innovative solutions to improve battery performance and extend battery life.
Introduction
It is appropriate to refer to lithium-ion batteries as a "technological wonder". They are the preferred option for many applications from a commercial standpoint, and lead-acid and nickel-metal hydride (NiMH) systems are being replaced by them more and more frequently. They also serve as a shining example of the fruitful outcomes of collaborative academic and industrial research.
Lithium-ion batteries are complex and multipart systems. According to Akira Yoshino, these batteries were created in 1986 as a result of successful safety testing of early prototypes. Since then, the performance of the batteries has greatly improved, with energy density and specific energy more than doubling. Lithium-ion cells are produced commercially, and manufacturing facilities produce between 1 and 10 GWh of energy annually. Cell prices have dropped due to mass production, decreasing from almost $5000 per kWh in 1991 to around $101 per kWh in 2021. Low-cost and high-energy-density cells initiated a period known as the "decade of the smartphone" after 2007. Demand has increased more than tenfold from 2011 to 2021. By 2030, demand is expected to reach 2-3.5 TWh and continue to grow rapidly. With lithium-ion technology becoming the standard, there are anticipated improvements in performance and reductions in battery industry prices.
What Exactly Are Lithium-ion Batteries and How Do They Operate?
The four essential elements of a lithium battery that you should be aware of are as follows:
- the lithium ions-containing electrolyte
- the separator, which permits lithium ions to travel through the battery but stops electrons from doing the same
- the cathode, which serves as a storage area for lithium ions prior to battery charging
- where lithium ions are kept until the battery discharges is the anode.
As the battery is being charged, lithium ions go from the cathode to the anode through the electrolyte. Ions then move back and forth from the anode to the cathode as the battery is in operation. By doing this, an electrical current is produced and moved from the current collectors to the electrical appliances in your house!
Changes in cell chemistry and design, pack engineering, and manufacturing procedures have improved cost and performance. In 1991, Sony began selling cells that used carbon-based "anodes" and lithium cobalt oxide (LiCoO2 or LCO) "cathodes," with the positive electrode active material containing 60% mass cobalt. In order to prevent confusion, we will henceforth refer to electrodes as "positive" and "negative" rather than the more commonly used phrases "cathode" and "anode," which are only appropriate for the discharge of rechargeable batteries.
A crucial ternary cathode component for lithium-ion batteries is lithium nickel cobalt manganese oxide, which has the chemical formula LiNixCoyMn1-x-yO2. More than two-thirds of the cobalt in lithium cobalt oxide is replaced by nickel and manganese, both of which are reasonably cheap. The cost advantage is clear to see. In terms of electrochemical performance and processing performance, lithium nickel cobalt manganate materials and lithium cobalt oxide materials are very similar to the other lithium-ion battery cathode materials, lithium manganate and lithium iron phosphate. As a result, nickel cobalt manganese oxide materials are quickly replacing lithium cobalt oxide as the favorite of a new generation of lithium-ion battery materials.
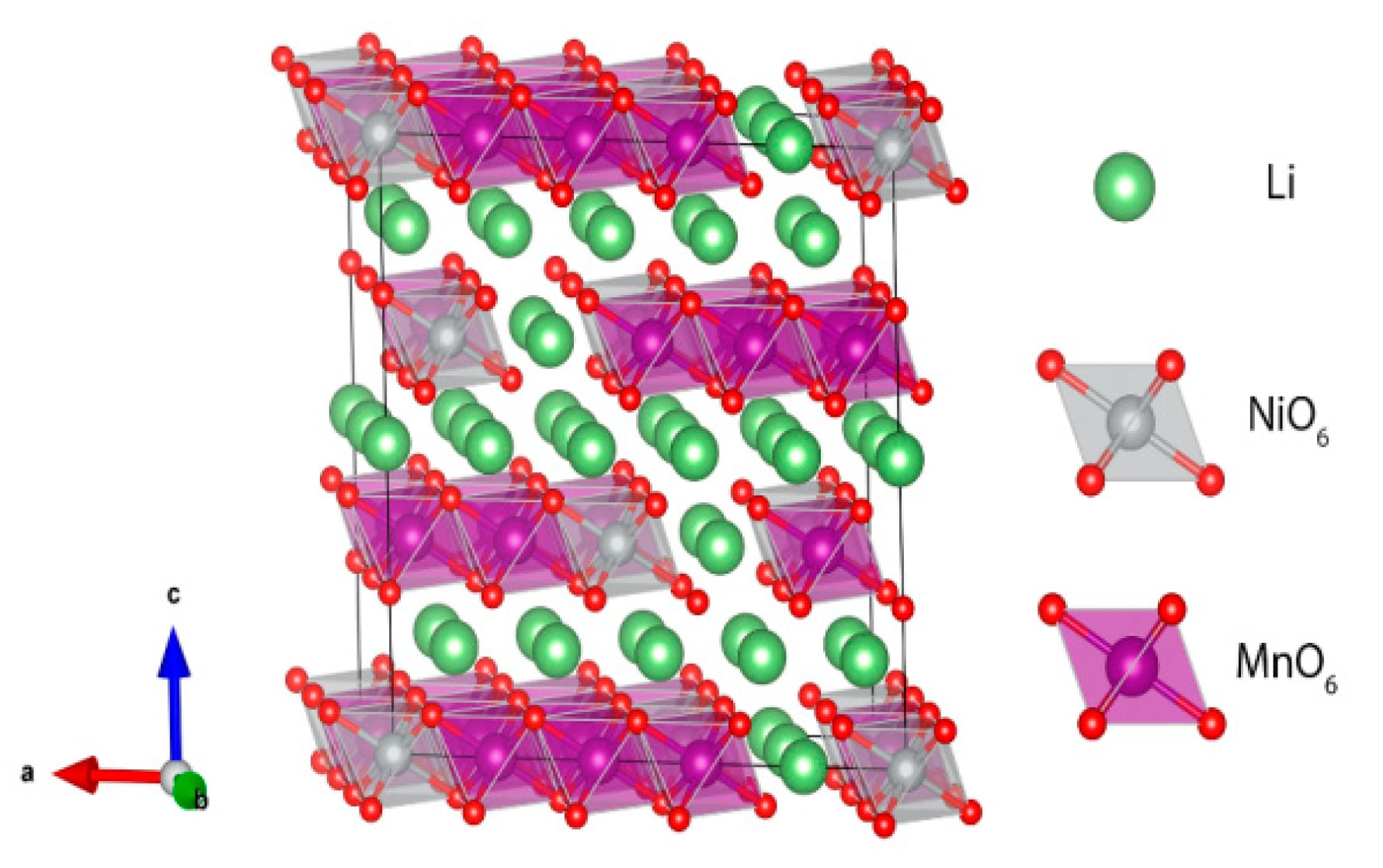
Figure 1. Crystal structure of Li-rich Li1.2Ni0.2Mn0.6O2.
Applications of Cathode Materials in Lithium-Ion Batteries
Consider power batteries, tool batteries, polymer batteries, cylindrical batteries, aluminum shell batteries, etc. as examples of applications for the cathode material in lithium-ion batteries.
Prospects for applications: Since its introduction, lithium nickel cobalt manganese oxide, an improved positive electrode material based on lithium cobalt oxide, has high capacity, strong thermal stability, and a wide range of charge and discharge voltage. The next generation of lithium-ion battery cathode materials are thought to be a suitable choice based on electrochemical performance, which has drawn considerable interest. Lithium nickel cobalt manganese oxide reduces the amount of cobalt, lowers the cost, and increases energy density by replacing a portion of the Co with Ni and the layered structure with Ni and Mn. In power-type cylindrical lithium-ion batteries, it has been widely utilized. To learn about the applications of Lithium Nickel Cobalt Aluminum Oxide (NCA) in lithium-ion battery, you can read our blog.
Nickel Lithium and Manganese Lithium, nickel, manganese, and cobalt are all combined metal oxides that make up the family of cobalt oxides. Although unstable, nickel is renowned for having a high specific energy. Despite having a low specific energy, manganese can create spinel structures that have a low internal resistance.
- NMC that is rich in nickel has a high discharge rate.
- Mn-rich compositions maintain greater thermal safety and cycle life.
- Excellent rate capability is provided by co-rich compositions.
These lithium-ion cell chemistries are referred to by the acronyms NMC or NCM.
Synthesis and Manipulation of Lithium Nickel Manganese Cobalt Oxide (NMC)
Lithium Nickel Cobalt Manganate is prepared primarily through co-precipitation and high temperature solid phase synthesis techniques. Lithium cobalt oxide, lithium hydroxide, nickel compounds, and manganese compounds are the main raw materials used. A precursor with a good ratio of lithium, manganese, cobalt, and nickel is produced using hydrothermal reaction; the precursor is then supplemented with a lithium source and processed to produce the precursor. To produce lithium, nickel, cobalt, and manganese oxide, the body is calcined. Battery materials must go over a fixed-line circulation path because of the mounting strain on the world's resources.
We, Nanografi, are dedicated to developing and improving our battery materials solution to provide our customers & business partners the most effective and efficient way.
For the creation of lithium-ion batteries with high energy densities, lithium nickel manganese cobalt oxide (NMC) cathodes are crucial. Currently, polycrystalline secondary particles—which are aggregated by anisotropic primary particles—make up the majority of currently available NMC products. The volumetric energy density, cycling stability, and production adaptability of the polycrystalline NMC particles have shown significant gravimetric capacity and good rate capabilities, however they do not meet expectations in these areas. Therefore, a different approach to the further development of high-energy-density batteries is suggested: well-dispersed single-crystalline NMC. The single-crystalline NMC product has been synthesized using a variety of methods, however the underlying mechanisms are still incoherent and disjointed.
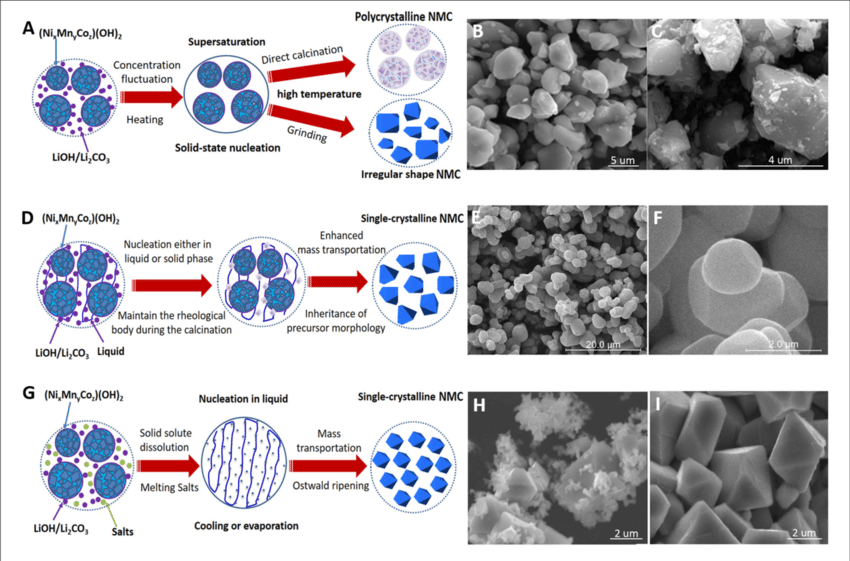
Figure 2. Production of Nickel Manganese Cobalt Oxide (NMC).
Growth Mechanism: NMC Cathode General Considerations
The production of three-dimensional nuclei from the supersaturated media (or matrix) and the growth of the nuclei into a bigger crystal entity are the two typical steps of crystallization. The new phase cannot form in the first step without supersaturation, which can be attained through concentration changes, solvent evaporation, and medium cooling. As long as the size of the nuclei can be greater than the crucial value R Mittemeijer.The decrease in free energy caused by thephase transition would outweigh the rise in surface free energy, and the nuclei would remain stable. It would dissolve into the medium if it didn't. When the mean distance between grains is large enough, mass transportation regulates the growth rate of crystals in the second stage. By lowering the total surface energy over time, the closed system tends to reach a minimal energy state where the Ostwald ripening (grain coarsening) process would predominate.
As a precursor medium, is typically combined with transition metal hydroxides, nitrides, and sulphates. The nucleation of NMC can be triggered by a small heat input above 200°C nevertheless, grain development is low until the calcination temperature surpasses the melting point of LiOH/Li2CO3. The mass movement and crystal formation are increased in the melts of Li precursors when the temperature during calcination exceeds 800°C. Since the homogeneous distribution of transition metal ions has typically been achieved by coprecipitation or milling and the NMC lattice formation primarily depends on oxygen and lithium migration, the slight evaporation of Li2O can further facilitate the mass transport and results in significantly accelerated crystal growth. In contrast, the phase and composition of NMC would change when calcined at 900-1,000°C due to vigorous lithium volatilization. Single-crystalline NMC cathode synthesis has received a lot of attention up to this point, and numerous techniques have been developed. These techniques can broadly be divided into three groups: solid-state reactions, solid-liquid reactions, and molten flux growth.
For more information about the synthesis and applications of lithium battery materials, visit our blog page.
NMC811 as a Novel and Promising Cathode Material for Lithium Batteries
Researchers are exploring anode-free Li metal batteries (LMBs) with liquid electrolytes for high energy density. However, challenges like reactivity of Li0 with the electrolyte, Li loss, and dendrite formation need solutions. The goal is an electrolyte that retains 80% capacity after 800 cycles, meeting EV demands. They tested 65 electrolyte combos, including co-solvents, additives, in a realistic setup with LiNi0.8Mn0.1Co0.1O2 cathode. Only 4 outperformed the baseline in total energy over 140 cycles, highlighting the complexities of liquid electrolytes for future LMB research.
The findings of an environmental evaluation of a Nickel-Manganese-Cobalt (NMC) Lithium-ion traction battery for Battery Electric Light-Duty Commercial Vehicles (BEV-LDCV) utilized for local and urban freight hauling were published in a study. The NMC111 Life Cycle Inventory (LCI) was provided, together with information on the operating and end-of-life stages and a Life Cycle Assessment of several NMC chemistries. Then, the environmental effects of the NMC111 battery's production processes were contrasted with those of a sodium-nickel-chloride (ZEBRA) battery. A diesel urban LDCV and two electric-battery LDCVs (powered by NMC111 and ZEBRA batteries, respectively) were analyzed in the second section of the work, taking a broad range of environmental impact categories into account. The outcomes demonstrated that the NMC111 battery had the highest production-related consequences across the majority of impact categories. The primary causes of the environmental impact were active cathode material, aluminum, copper, and energy use for battery production. When vehicle application was examined, NMC111-BEV demonstrated lower environmental impacts than ZEBRA-BEV across all impact categories. This was mostly brought about by the NMC111 battery's higher operating efficiency. The electric powertrains also outperformed the diesel one in terms of global warming, abiotic depletion potential from fossil fuels, photochemical oxidation, and ozone layer depletion when compared to a diesel LDCV. In nearly every other evaluated impact category, the diesel LDCV fared better.
Life Cycle Assessment of NMC811
The NMC 811 battery is an enhancement over current market offerings rather than a fresh and distinctive battery type. According to the various dosage ratios of nickel, cobalt, and manganese components used in the battery cathode, this foundation is made up of the common lithium battery. 111 type, 523 type, 622 type, 811 type, etc. are further divisions. The cathode of the 811 is made up of 80% nickel, 10% cobalt, and 10% manganese.
Materials
The great energy density of the NMC 811 battery is its most important characteristic. This results from the cathode's nickel, which can raise material activity and, as a result, energy density. Additionally, the high nickel concentration is crucial for boosting capacity. Additionally, cobalt is an active component that strengthens the material's laminar structure and raises its discharge capacity. The electrodes' supporting manganese component, which contributes stability during charging and discharging. The energy density of Grepow's NMC 811 battery, for instance, is 275 Wh/Kg.
Features
The energy released per unit mass, measured in Wh/kg, is called weight energy density. A battery with a 275Wh/kg rating may discharge 275Wh of power per kilogram. A 10w bulb in your home may be kept lighted for 27.5 hours using, for instance, 275Wh, which is comparable to 0.275kWh. As a result, the NMC 811 offers more energy from batteries of the same caliber. It's wonderful to have a high energy density because it makes the battery's weight smaller, which is important for UAVs. Utilizing the NMC 811 battery really results in a 15% weight reduction, a 30% increase in operating duration, and lower maintenance expenses.
Test Data
According to the test chart contrasting the Grepow NMC 811 battery and the LCO battery, the NMC 811 battery has a long-life cycle of over 600 cycles. At the same time, both batteries were drained to 3.0V at a 5C rate and charged to 4.2V at a 1C rate. The NMC 811 battery's capacity retention rate was 93% at the 500th cycle, which was higher than the LCO batteries.
Lithium Nickel Manganese Cobalt Oxide vs Lithium Iron Phosphate
Despite being more common than ever, lithium-ion solar batteries are not all created equal. And we're not just talking about variations in brand here; the materials the batteries are actually constructed of can have a significant impact on how well they work. Lithium iron phosphate (LFP) and nickel manganese cobalt (NMC), the two most common forms of lithium-ion batteries for solar storage, are both good choices for homeowners who want to store the energy their solar systems produce.
Important information regarding NMC and LFP batteries:
- Both NMC and LFP batteries are lithium-ion battery varieties.
- As an excellent alternative for solar energy storage in the house, nickel manganese cobalt (NMC) batteries include a cathode consisting of a combination of nickel, manganese, and cobalt.
- Batteries made of lithium iron phosphate (LFP) have a lithium iron phosphate cathode.
- The lithium-ion chemistry you select for your battery installation is less significant than selecting a qualified, certified installer.
NMC battery
A particular kind of lithium-ion battery is an NMC battery. The cathode of NMC batteries is composed of nickel, manganese, and cobalt. NMC batteries are probably used more frequently than you know to power electric vehicles, laptops, and smartphones. They're also one of the most often used solar storage alternatives.
Due to their longer lifespan, increased energy storage capacity, and low maintenance requirements, NMC batteries have surpassed lead acid batteries as the most preferred option for solar storage. In fact, NMC batteries are among the most widely used solar batteries on the market right now.
LFP battery
The cathode of LFP batteries is made of lithium iron phosphate (LiFePO4). Compared to some of the materials used in NMC batteries, primarily cobalt, the iron and phosphate used to form the cathode are both more accessible and less expensive.
Comparison
When looking for a solar battery, there are a few important factors to think about, including the battery's performance, longevity, safety, price, and overall value.
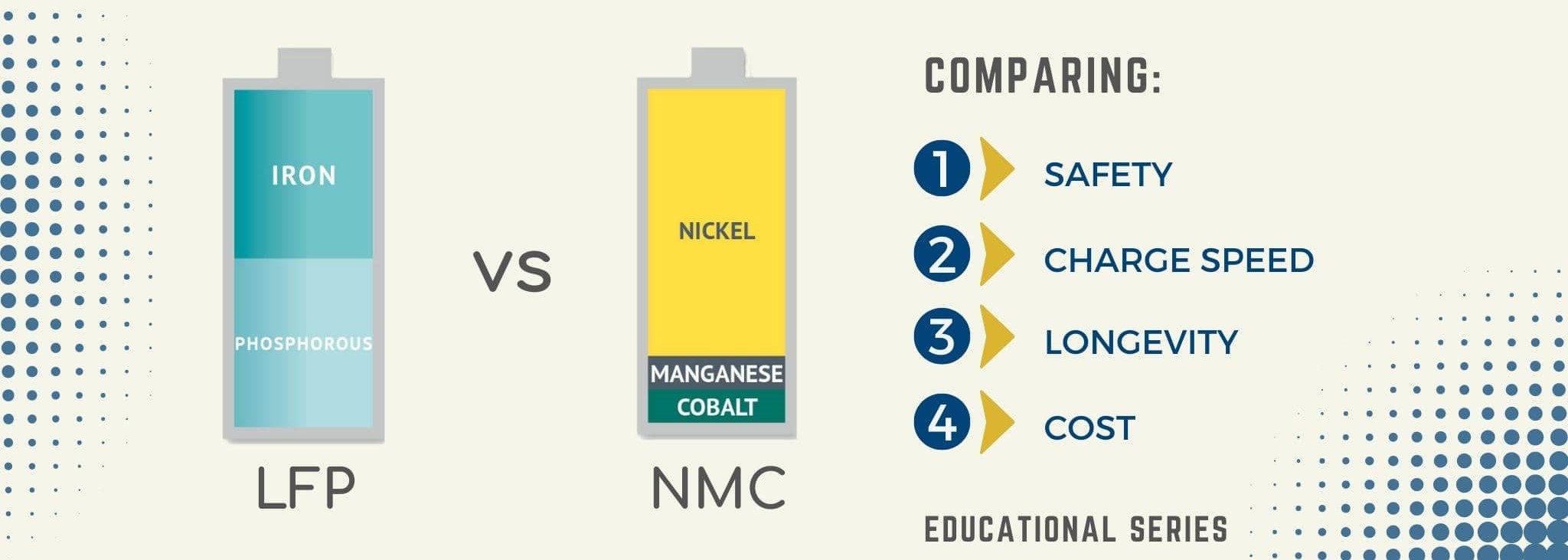
When compare the strengths and weaknesses of NMC and LFP batteries:
Performance: The overall performance of NMC and LFP batteries is quite similar. Both varieties come in a range of sizes, ranging from 3 kWh to over 20 kWh. The average homeowner only needs about 10 kWh of storage, which both options will provide.Having said that, the two have a few minor distinctions. Compared to LFPs, NMCs are slightly more efficient, work a little better when the state of charge is low, and can resist colder temperatures. However, if your battery is put inside or if the temperature in your location doesn't fluctuate greatly, you probably won't need to be concerned about this. Additionally, NMC batteries are physically smaller than LFP batteries of the same capacity due to their better energy density. Although homeowners typically aren't concerned about this, if you do have a small space, you might want to think about using an NMC battery.
Lifespan: A battery loses some of its capacity to store a charge as you use it. NMC batteries degrade more slowly than LFP batteries, therefore they can store and discharge more electricity over time. Remember that the battery's quality will also affect how long it lasts; therefore, before making a choice, you might wish to review the product guarantee. A low-cost, shoddy-built NMC battery might not outlive a premium LFP battery. But as long as you get a reliable installer, you won't likely encounter many subpar batteries.
Safety: The safety of an NMC battery is among its main advantages. At higher temperatures, nickel manganese cobalt is a more stable compound than lithium iron phosphate. Additionally, NMC batteries are better able to manage higher power pulls. NMC batteries are therefore less susceptible to thermal runaway. NMC batteries are less prone to catch fire than LFP batteries, to put it briefly. An LFP battery is more susceptible to problems if it is handled incorrectly or under excessive stress. To reduce the likelihood of anything going wrong, it's crucial to have your batteries installed by a licensed, reputable professional.
A one-time fee: Compared to LFP batteries, NMC batteries are often a bit less expensive. Economies of scale play a major role in this; NMC batteries are more widely used in the United States, which results in slightly lower pricing. A little more work may be required to transfer and install LFP batteries because of their somewhat larger size. Simply because they are larger, the cabinets that house LFP cells could require more materials. Larger-scale projects undoubtedly take into account the cost differential between NMC and LFPs more. Both forms of chemistry are often available for use in household solar systems.
Value: NMC batteries are less expensive upfront and offer a little bit more value, as we just stated. In order to fully assess which battery offers the best value, it's vital to take into account how much power it can deliver. Because of its longer lifespan, NMC batteries frequently prove to be the superior choice.
Conclusion
NMC (Nickel Manganese Cobalt) Oxide holds significant promise as a material for lithium-ion batteries. This compound offers numerous advantages such as high energy density, long cycle life, low self-discharge rate, and thermal stability due to the combination of different metal cations. With its superior electrochemical properties, NMC has broad application potential in areas like electric vehicles, portable electronic devices, and energy storage systems. Future research should focus on to further enhance the performance, reliability, and sustainability of NMC. As a result, this material will continue to remain an exciting candidate for the advancement of lithium-ion technology. We, Nanografi, are working to present our products to you in a high quality and innovative way suitable for the developing world of nanotechnology. Visit Nanografi to review our products.
References
Abdel-Ghany, A., Hashem, A. M., Mauger, A., & Julien, C. M. (2020). Lithium-Rich Cobalt-Free Manganese-Based Layered Cathode Materials for Li-Ion Batteries: Suppressing the Voltage Fading. Energies 2020, Vol. 13, Page 3487, 13(13), 3487. https://doi.org/10.3390/EN13133487
Accardo, A., Dotelli, G., Musa, M. L., & Spessa, E. (2021). Life cycle assessment of an NMC battery for application to electric light-duty commercial vehicles and comparison with a sodium-nickel-chloride battery. Applied Sciences (Switzerland), 11(3), 1–32. https://doi.org/10.3390/APP11031160
Battle of the Batteries: NMC vs LFP. Which is Best for Solar? (n.d.). Retrieved February 20, 2024, from https://www.solarreviews.com/blog/lithium-ion-solar-batteries-compared
Frith, J. T., Lacey, M. J., & Ulissi, U. (2023). A non-academic perspective on the future of lithium-based batteries. Nature Communications 2023 14:1, 14(1), 1–17. https://doi.org/10.1038/s41467-023-35933-2
Hydra Skyscraper- eVolo | Architecture Magazine. (n.d.). Retrieved February 20, 2024, from https://www.evolo.us/hydra-skyscraper/
LFP vs NMC: Best Battery for Energy Storage? - TROES Corp. (n.d.). Retrieved February 20, 2024, from https://troescorp.com/lfp-vs-nmc-best-battery-for-energy-storage/
Lithium Nickel Cobalt Aluminum Oxide (NCA) in Lithium-Ion Battery Applications - Nanografi Nano Technology. (n.d.). Retrieved February 20, 2024, from https://nanografi.com/blog/lithium-nickel-cobalt-aluminum-oxide-nca-in-lithiumion-battery-applications/
Lithium Nickel Manganese Cobalt Oxides - Battery Design. (n.d.). Retrieved February 20, 2024, from https://www.batterydesign.net/lithium-nickel-manganese-cobalt-oxides/
Properties and Applications of Lithium Battery Materials - Nanografi Nano Technology. (n.d.). Retrieved February 20, 2024, from https://nanografi.com/blog/properties-and-applications-of-lithium-battery-materials-/
Self-healing concret... - Wikipedia. (n.d.). Retrieved February 20, 2024, from https://en.wikipedia.org/wiki/Self-healing_concret...
Schematic view of the crystal structure of Li-rich Li1.2Ni0.2Mn0.6O2.... | Download Scientific Diagram. (n.d.). Retrieved February 20, 2024, from https://www.researchgate.net/figure/Schematic-view-of-the-crystal-structure-of-Li-rich-Li12Ni02Mn06O2-The-red-green_fig1_342722995
Wang, T., Ren, K., He, M., Dong, W., Xiao, W., Pan, H., Yang, J., Yang, Y., Liu, P., Cao, Z., Ma, X., & Wang, H. (2020). Synthesis and Manipulation of Single-Crystalline Lithium Nickel Manganese Cobalt Oxide Cathodes: A Review of Growth Mechanism. Frontiers in Chemistry, 8, 575356. https://doi.org/10.3389/FCHEM.2020.00747/BIBTEX
What is the NMC 811 Battery? What are its features? | Battery Monday. (n.d.). Retrieved February 20, 2024, from https://www.grepow.com/blog/what-is-the-nmc-811-battery-what-are-its-features-battery-monday.html
ZEBRA battery - Wikipedia. (n.d.). Retrieved February 20, 2024, from https://en.wikipedia.org/wiki/ZEBRA_battery
Recent Posts
-
MXenes from MAX Phases
MXenes, a group of two-dimensional materials derived from MAX phases, are gaining significant tracti …19th Oct 2024 -
Preserving History with Graphene's Power
Cultural artifacts are at risk of deterioration over time due to the destructive effects of both na …11th Oct 2024 -
The Role of Graphene in Neuroelectronics
Neuroelectronics is an interdisciplinary field that aims to develop devices that can interact with t …4th Oct 2024