Nanotechnological Viewpoint in COVID-19 Treatment
The world is now under the invasion of the highly lethal infectious Coronavirus overtly and covertly with more than one and a half million infected and thousands of officially confirmed death cases. The announcement of COVID-19 as a global pandemic after the shift in the epicenter of the outbreak and the consequent state of emergency in almost all four corners of the world have obliged most research projects across the world and high profile universities to be suspended in order to find the fastest and most effective way to get over the extremely critical situation caused by the deadly outbreak. In addition to the scientific community, businesses have stopped the manufacturing of their routine products and concentrated on producing masks, alcohol, sanitizing solutions and gels in order to be responsive to the practically huge demand for the most essential and basic tools to fight the coronavirus. The upcoming sections are mainly discussing the use of nanoparticles in creating vaccines that have been previously used in treating infectious diseases caused by pathogens from Coronavirus family as well as the potential nanoparticle-based vaccines and drugs to cure the novel COVID-19.
Click Image to Reach Our Other Articles About COVID-19
Vaccine Based on Poly-(Lactic-Co-Glycolic) Nanoparticles
Today, finding a vaccine as a global race is regarded the very first priority for most governments and a huge concern for people everywhere. Previously in the case of Middle East respiratory syndrome coronavirus (MERS-CoV), there were calls in order to develop a novel and specific vaccine capable of offering secure and efficient prophylactic measures. More specifically in this issue, a new vaccine based on the application of poly-(lactic-co-glycolic acid) (PLGA) nanoparticles capable of delivering STING agonists and subunit viral antigen was developed. Stimulator of interferon genes (STING) are originally proteins and a new class of cancer drugs regarded as a part of a signaling pathway responsible to regulate the molecules involved in the innate immune response. Based on this nanotechnological vaccination procedure, STING agonists are embedded in capsid-like polymeric nanoparticles with a hallow morphology. These polymeric nanoparticles possess clear local immune activation and pH-responsive release profile as well as reduced systematic reactogenecity. Upon the conjugation of the antigen, the hollow polymeric nanoparticles deliver the similar morphology to the virus in order to facilitate the delivery of the STING and antigens to the immune cells and lymph nodes 1.
Vaccine Based on Spike Protein Nanoparticles
Another vaccine to treat MERS coronavirus is by spike protein nanoparticles. To do so, spike protein nanoparticles and a recombinant adenovirus serotype 5 encoding the MERS-CoV spike gene are allowed to interact with aluminum adjuvant. Later and based on the heterologous prime–boost vaccine strategy, the vaccine is capable of inducing some particular immunoglobulin G against MERS-CoV. However, neutralizing antibodies are induced through homologous immunization as well as heterologous prime–boost immunization with spike protein nanoparticles against MERS-CoV. In this case, the activation of Th1 cell is done by adenovirus serotype 5 encoding the MERS. As a result, the heterologous prime–boost could lead to immune responses that last longer against the MERS-CoV 2.
Click Image to Find Out More About Nanomaterials in Medicine
Vaccine Based on Virus-like Particle Mimetic Nanovesicles
In this method towards creating a vaccine to fight MERS-CoV, the proteins found in the MERS-CoV structure are expressed in silkworm larvae and Bm5 cells in order to develop potential vaccines. The spike protein of MERS-CoV that originally lacks transmembrane cytoplasmic domains (SΔTM)is purified to be embedded in the hemolymph of silkworm larvae taking the advantage of a bombyxin signal peptide. Later, the purified SΔTM engages in small nanoparticles and spike protein formation. In addition to this, SΔTM can bind to human dipeptidyl peptidase 4 (DPP4). The co-expression of spike proteins is carried out in MERS-CoV membrane protein (M), envelope protein (E) and the Bm5 cells outside the cell forming MERS-Coronavirus-like particles (MERS-CoV-LPs) 3.
Nanomedicine Insight into Chloroquine to Fight COVID-19
The results from a recent clinical cell culture studies demonstrate that chloroquine (the 70-year-old malaria drug) could potentially serve as an efficient therapeutic agent against coronavirus disease 2019 (COVID-19). With its derivative hydroxylchloroquine, chloroquine is known to be an inexpensive and safe drug to be applied as a prophylactic strategy to treat malaria and the common autoimmune diseases. It should be noted that eye damage is the most common side effect of chloroquine in a long term. There is a fact that chloroquine’s anti-viral mechanism is still disputed. Nevertheless, studies show that it has effective therapeutic activity against viruses such as SARS-CoV in and human coronavirus OC43 in cell culture studies. In nanomedicine, chloroquine has been used to investigate the absorption on nanoparticles in cells in order to get a comprehensive view of cells interactions with nanoparticles when chloroquine is present. Such interactions might clarify the mechanisms that are in progress exactly at the early stages before the viral replication takes place. Nanomedicine can particularly provide enough information concerning the alterations in coronavirus (SARS-CoV-2) uptake in cells 4. Figure 1 shows the mechanism through which chloroquine exerts therapeutic impact against COVID-19.
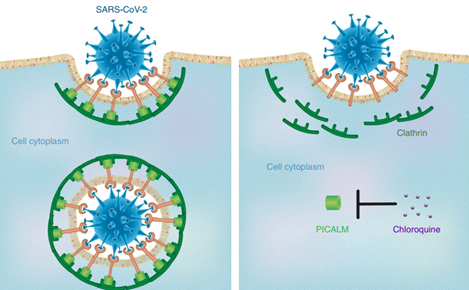
Figure 1. Therapeutic mechanism of Chloroquine against COVID-19 4
Chemically speaking, chloroquine is regarded a weak alkaline drug that gets encapsulated in organelles with low pH and enclosed membrane and alters their acidity. In mammalian cells, chloroquine treatment results in the pH increase of lysosomes. Through preventing lysosome of fusion, the lysosome interferes the upstream endocytic trafficking with a consecutive phenomenon like a traffic jam to block the transportation from cell membrane. It is speculated that chloroquine’s antiviral impact is through inhibiting viral fusion and replication (which are considered pH dependent), preventing the host receptor protein glycosylation along with the viral envelope glycoprotein 4.
Nanoparticles Endocytosis Inhibition by Chloroquine
Studies have demonstrated that chloroquine inhibits nanoparticles endocytosis using some resident macrophages due chloroquine’s broad spectrum quality. Relevant clinical doses of chloroquine causes monodispersity of synthetic nanoparticles with various sizes ranging from 14 to 2600 nm and shapes in cells and within mice mononuclear phagocyte. They studies concerning the mechanism of chloroquine in treating the COVID-19 reveal that it declines the expression of a protein called phosphatidylinositol binding clathrin assembly (PICALM) which is considered as one of the most common proteins in clathrin-coated pits. As a cargo-selecting clathrin adaptor, PICALM engages in sensing and deriving the membrane curvature regulating the endocytosis rate. PICALM depletion causes the inhibition of clathrin-mediated endocytosis as the responsible predominant pathway to internalize nanoparticles 4. Based on the data from the particle characterization techniques, coronavirus (SARS-CoV-2) size falls in the range of 60 to 140 nm and is spherical morphologically. This means any mechanism to mediate the impact of chloroquine against coronavirus causes a decrease in cells ability to perform clathrin-mediated endocytosis of nanoparticles structure because of suppressing PICALM.
References
1. Lin, L. C. W. et al. Viromimetic STING Agonist-Loaded Hollow Polymeric Nanoparticles for Safe and Effective Vaccination against Middle East Respiratory Syndrome Coronavirus. Adv. Funct. Mater. 29, 1–15 (2019).
2. Jung, S. Y. et al. Heterologous prime–boost vaccination with adenoviral vector and protein nanoparticles induces both Th1 and Th2 responses against Middle East respiratory syndrome coronavirus. Vaccine 36, 3468–3476 (2018).
3. Kato, T., Takami, Y., Kumar Deo, V. & Park, E. Y. Preparation of virus-like particle mimetic nanovesicles displaying the S protein of Middle East respiratory syndrome coronavirus using insect cells. J. Biotechnol. 306, 177–184 (2019).
4. Hu, T. Y., Frieman, M. & Wolfram, J. Insights from nanomedicine into chloroquine efficacy against COVID-19. Nat. Nanotechnol. 19–21 (2020) doi:10.1038/s41565-020-0674-9.
Recent Posts
-
Advanced Materials for Unmanned Aerial Vehicle (UAV) Protection Against Laser
Consider a UAV on a critical mission, rendered inoperative by a sudden laser attack. With the increa …26th Jul 2024 -
Simulation and Modeling of Material Properties
Our world is composed of a dazzling array of materials, each with its own unique properties that dic …19th Jul 2024 -
Advanced Coatings for Superior Corrosion and Wear Resistance
Corrosion and wear pose significant challenges across various industries, leading to substantial eco …12th Jul 2024