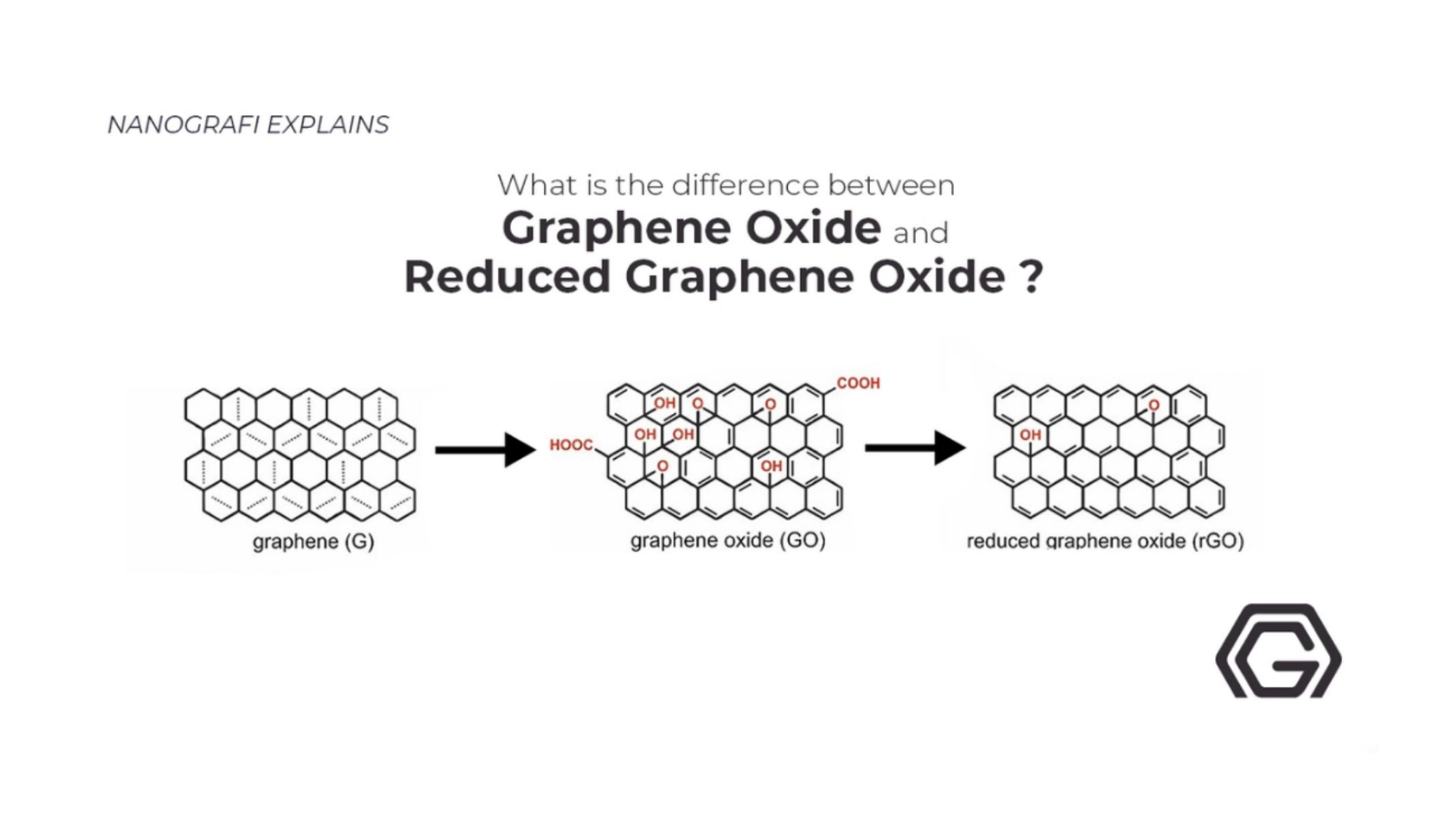
Explained: Graphene, Graphene Oxide, and Reduced Graphene Oxide and Applications
Graphene is a very interesting material. It is a 2-dimensional sheet that is formed of carbon atoms. Graphene has certain characteristics as at 0.77 mg/square meter, it is very light and strong. Although graphene is a semi-metal substance, yet it possesses high electrical conductivity. Graphite oxide can be turned into reduce graphite oxide. The quality of reduced oxide is less than graphene. Artificial graphite oxide can be formed by treating graphite with a strong oxidizer.
Offerman and Hummers are the methods that are used to produce graphite oxide. The chemical properties of graphene oxide and graphite oxide are almost the same but their structures are very different. The two most used methods for the conversion of graphite into graphene are utilizing stirring and signification.
Introduction
In the past scientists have applied different methods for the creation of GO from rGO. rGO has been used in composite materials, energy storage, and field-effect transistor. GO and rGO have been used in gas sensing applications. Because of the high conductivity, rGO has gained much attention and due to good sensing abilities GO has gained attention. Because of high electrical conductivity, cyclic stability and specific surface area rGO is a nice option for super capacitance. GO and rGO possess anti-bacterial characteristics. GO also has biomedical applications.
Graphene oxide
Graphene oxide is graphene’s form. Oxygen functional groups are included in it, and it has remarkable characteristics that can differ from the characteristics of graphene. These oxidized functional groups are eliminated by lessening graphene oxide for attaining a graphene material. Reduced graphene oxide is the name of this graphene material, it is usually referred to as rGO. Graphite oxide can also give rGO. It is a material that is made of a combination of many graphene oxide layers, after reduction’s series to graphene oxide and rGO.
Graphene’s Characteristics
Mechanical characteristics
The inherent strength of graphene is one of its other excellent properties. At 0.77 milligrams per square meter, graphene is extremely light and extraordinarily strong. Paper’s 1 square meter is 100 times higher, roughly. Usually, it is said that graphene’s single sheet of one atom thickness, is enough in size for covering a complete football field, while weighing under 1 single gram.
Electronic characteristics
One of graphene’s most beneficial characteristics is that it’s a semimetal with zero-overlap (with both electrons and holes as charge carriers) with extremely high electrical conductivity. 6 electrons in total are possessed by the carbon atoms, 4 are contained in the outer shell and the inner shell contains 2. Pi (π) electrons are the highly-mobile electrons. The location of the Pi electrons is below and above the graphene sheets, and they overlap. The overlapping of these pi orbitals aid in improving graphene’s carbon to carbon bonds. The anti-bonding (the conduction and valence bands) and the bonding of these pi-orbitals fundamentally dictate graphene’s electronic characteristics.
Optical properties
It is an interesting and rare property of graphene that it has the capability of absorbing a large 2.3% white light, particularly taking into consideration that its thickness is only 1 atom. This property of graphene is because of its already mentioned electronic characteristics; the electrons function like massless charge carriers with extremely high mobility. It was proved some years back that the Fine Structure Constant determines the amount of white light that’s absorbed, instead of being dictated by the material specifics.
How is rGO formed?
Graphene oxide’s (graphite oxide) reduction to rGO is attractive and famous as the cheap and effective ways of making graphene (rGO) are being intensively sought. There is an existence of various methods of reduction, and they are simple and cost-efficient.
Defects
rGo has the same characteristics as graphene and it is graphene’s form (good conductive characteristics etc.). Usually, more defects are contained by rGO and it is of lower quality as compared to graphene that is made from graphene directly. Residual oxygen and other heteroatoms along with structural defects are contained by the reduced graphene oxide (rGO). rGO is considered an interesting material that is enough for numerous applications in quality, but more attractive manufacturing processes and pricing. However, rGO is still considered to have a less than perfect resemblance with pristine graphene. One can use reduced graphene oxide for the same numerous applications that are appropriate for the usage of graphene, for instance, sensors, conductive inks, and composite materials. It all depends on the quality of the specific material.
Natural choice
It is them having ease in producing enough graphene’s quantities at a comparatively low cost that makes the reduced graphene oxide an understandable and natural choice for applications calling for the material’s large amounts. The process of making reduced graphene oxide is very significant as a large influence is made by it on the produced-rGO’s quality, and thus will determine the range of closeness rGO will come to pristine graphene, regarding characteristics and structure.
Number of processes
GO can be reduced and there are various processes to do that, based on electrochemical, thermal, or chemical approaches. High-quality rGO like high-quality graphene can be produced by using some of these methods but carrying them out can be time-consuming, expensive, or complex. There are various approaches to functionalizing the material in various applications for particular usages once the production of reduced graphene oxide is completed. We can improve the compound’s characteristics for suiting commercial applications by combining rGO with other 2-D materials to create new compounds or by treating rGO with numerous chemicals. GO’s a reduction to rGO is performed in some applications as a device manufacturing process’s part.
Example
For instance, GO can start a process, if it is mixed with material for creating a composite, and lessen GO as a part of the composite creation process or afterward into rGO.
Graphite Oxide
It is a compound that contains oxygen, hydrogen, and carbon molecules. Graphite is treated with strong oxidizers like sulphuric acid for the artificial creation of graphite oxide. These oxidizers react with the graphite and eliminate an electron in the chemical reaction. Such kind of reaction can also be called a redox reaction, as the reactant is oxidized and the oxidizing agent is reduced.
Hummers and Offerman method
In the past, the Hummers and Offerman methods have been the most usual method to create graphite oxide. In this method, a mixture of potassium permanganate (an extremely strong oxidizer), sodium nitrate, and sulphuric acid, for treating graphite. Although recently there has been a development of the other methods that are more efficient according to the reports, passing 70% of the oxidization levels, via utilization of increased amount of potassium permanganate, and instead of adding sodium nitrate, it involves the addition of the phosphoric acid combined with the sulphuric acid.
Oxidization
The oxidation has graphene oxide as its by-product as when graphite reacts with the oxidizing agent m, the interlayer spacing between graphite’s layers is increased. Then the oxidized compound can disperse in a base solution like water, and produce graphene oxide.
Difference between graphene oxide and graphite oxide
There are a lot of chemical similarities between graphene oxide and graphite oxide but they are extremely different when it comes to structuring. Water intercalation between the compound’s atomic layers due to water intercalation causes the interplanar spacing between the compound’s atomic layers and that is the major difference between the graphene oxide and graphite oxide. The oxidation process causes this increased spacing and it disrupts the sp2 bonding network too, which shows that both graphene oxide and graphite oxide are usually known as electrical insulators.
Graphite Oxide to Graphene Oxide
Individual graphene layers can be extremely damaged from the process in which graphite oxide is turned into graphene oxide, and when further lessening the compound, it has more consequences. More damages are undesirable as the Individual graphene platelets are already damaged by graphite’s oxidization process to graphite oxide, thereby lessening their mean size. Flakes of a few layers and monolayer graphene are contained by the Graphene oxide, interspersed with water (depending on the base media, surface functionality can weaken the platelet to platelet interactions, resulting in enhanced hydrophilicity).
Possible methods for the conversion
There are some possible methods for turning graphite oxide into graphene oxide. Utilizing stirring, sonication, or both’s combinations are the most usual methods. An extremely time-efficient way to exfoliate graphite oxide is sonication, and its success rate in exfoliating graphene is extremely high (almost too full exfoliation levels), but graphene flakes can also be heavily damaged by it, lessening them in surface to nanometres from microns, and also forms a broad range of graphene platelet sizes. Mechanical stirring can take a lot of time for accomplishing and it is a much less heavy-handed method.
From Graphene Oxide to Reduced Graphene Oxide
Producing reduced graphene oxide by reducing graphene oxide is a very major and critical process as it possesses a large influence on the produced-rGO’s quality, and this will determine the closeness that rGO will come to pristine graphene, regarding structure. rGO is the clearest solution in large-scale operations too because it has comparatively eased in forming graphene’s enough quantities.
To get more information about the difference between Graphene Oxide (GO)
and Reduced Graphene Oxide (rGO),
you can read our blog post here.
Numerous reduction ways
The reduction can be attained in a different number of ways, although all of them are methods that are based on electrochemical, thermal, or chemical means. Extremely high-quality rGO like pristine graphene can be produced by using some of these techniques, but carrying them out can be time-consuming or complex
Scientists have made GO from rGO in the past by
- Using hydrazine hydrate to treat GO and maintaining the solution for 24 hours at 100.
- Exposure of GO for some seconds to the hydrogen plasma
- Exposure of GO to strong pulse light’s another form, like those that the xenon flash tubes make.
- Heating the GO at various degrees in distilled water for various lengths of time.
- Linear sweep voltammetry
- Combining GO with an expansion-reduction agent like urea and then a solution will be heated for the area for the release of reducing gases, after cooling.
- Heating GO directly in the furnace to extremely high levels.
Structure
GO is also used by the rechargeable batteries in their structure. GO material’s high specific surface area is essential for these materials' high capacity like rGO. In addition, active bonding areas are offered by the oxygen-containing compounds on the surface of GO for electrochemical materials. It is because of the production of solid electrolyte interphase (SEI) and Lithium ions reaction with the oxygen functional groups that give GO-based anodes its poor cycling capacity even though changing the concentrations of the oxygen-containing compounds can tune the GO’s electrical characteristics. GO has been successfully utilized in the cathode materials however it exhibited poor performance in Li-ion batteries anode materials. For instance, the GO/LiFeSO4F composite functions as a cathode material for enhanced rate capability and cycle stability for LiBs.
rGO Applications
It can be generally said that rGO can do what graphene does because the characteristics of both of these materials are very much alike, however at the rGO end, albeit is less impressive. Specific rGO’s chemistry and resulting morphology along with the preparation method determine the rGO’s characteristics. One can use the reduced rGO for various applications and among those applications are field-effect transistors, composite materials, energy storage, and more.
Energy Storage
rGO is used for energy storage applications in lithium-oxygen, lithium-sulfur, and lithium-ion batteries. This material’s high surface area is a significant benefit for attaining high-capacity energy storage devices. These rGO materials extremely conductive nature are especially used in the rechargeable batteries’ cathode and anode materials. The exchange of electrons and effective ion transfer is promoted by the conductive carbon network.
Rate capability
Moreover, the rate capability can be enhanced by the Li’s high diffusivity on the graphene planes. For instance, rGO platelets were decorated by Fe2O3 nanoparticles and were utilized for lithium-ion batteries as an anode material that displayed 1693 and 1227 mAh g-1 of discharge and charge capacities. rGO electrode was under various studies and they exhibited that when it comes to high capacity energy storage applications, rGO is a convenient material.
Cathode material
In lithium-ion batteries, free-standing rGO films are successfully utilized as the cathode material. High-capacity cathode electrodes require a high content of functional oxygen-containing compounds in the structure of rGO.
Lithium-sulfur batteries
In lithium-sulfur batteries, GO composites are used to develop high-energy density batteries. For instance, lithium and sulfur polysulfide’s can use reactive functional groups 9’ the GO materials to immobilize themselves on the GO materials. Lithium-sulfur cells are enabled by the strong interaction between sulfur and GO or polysaccharides. They displayed stable cycling for more than 50 deep cycles and 950–1400 mAh g-1 of high reversible capacity.
Solar Cells
rGO and GO’s large specific surface area is important and exciting characteristics for solar cells applications. GO is used in organic photovoltaics as an effective interfacial layer (IFL) and is also utilized as the hole transport and electron blocking layer because of its semiconducting characteristics. Under environmental and thermal stress, GO majorly improves the durability of the device by increasing the active layer-IFL interfacial stability.
Cathode materials
GO is additionally used in dye-sensitized solar cells’ cathode materials in combination with rGO composite’s counter electrodes like multiwall carbon nanotube-rGO nanoribbon and rGO–TaON composite.
Sensors
There has been the utilization of both rGO and GO in gas sensing applications. Due to their high electrical conductivity and surface area, rGO gains attention whereas due to its high and active surface area, GO displays good sensing capabilities. rGO/CuFe2O4 nanocomposite is used for a high–performance NH3 gas sensor which utilizes CuFe2O4 sensing ability and rGO’s conductivity. On the other hand, cuprous oxide and graphene oxide (GO/Cu2O) nanocomposite-based sensors are utilized for trimethylamine (TMA) gas sensing. Good stability, selectivity, reversibility, and sensitivity are shown by the system in 60 days. Both GO and rGO nanocomposites are good sensors for humidity, nitrogen dioxide, and hydrogen.
Supercapacitors
Supercapacitors function based on electrochemical double-layer capacitance (EDLC) and release energy -5 the electrochemical interface by nanoscopic charge separation between an electrolyte and an electrode. rGO is a good option for the Supercapacitors applications of a new generation because of rGO’s cyclic stability, specific surface area, and high electrical conductivity.
Super capacitor electrodes
Supercapacitors electrodes are developed by using several different rGO nanocomposites like rGO/ZnO, rGO-carbon black, and rGO/Zn/PCz. These Supercapacitors have enhanced the capacitance to up to 33.80 F/g, leading to high energy (E= 1.66 Wh/kg) and power- (P = 442.5 W/kg) storage capabilities. Additionally, reduced graphene oxide further increases the surface area and Supercapacitors capacitance consequently and it is utilized for Supercapacitors applications in the form of aerogel. Although, the studies on GO are less because of the GO material’s lower electrical conductivity as compared to rGO.
Membranes
Graphene oxide’s porous structure and chemically active nature can be used to enhance the characteristics of the membrane and the separation performance. People used GO-polymer composites for CO2/N2 and O2/N2 separation applications. Furthermore, the membrane’s mechanical characteristics can enhance by including GO into the structure of the membrane.
Biosensors
GO and rGO’s composites are utilized as field-effect transistors (FET), electrochemical, and optical biosensors. Usually, metal nanoparticles like silver or polymers and platinum are contained by these composites. Biocompatible rGO and GO have been exploited in a broad range to sense biomolecules like arbitrary DNA mutation, optical aptamer, multiplexed microRNA, DNA/RNA aptamer, microRNA, D-glucosamine, DNA, and glucose.
Optical biosensing
GO material’s fluorescent behavior is utilized for the applications of optical biosensing for detecting various biological molecules like metal ions, food toxins, NAs, dopamine, H2O2, and cancer biomarkers glucose.
Biomedical Applications
Remarkable biocompatibility and DNA adsorption characteristics are shown by GO. According to findings, the binding of DNA to GO is extremely reversible and stable. Characteristics like these make the preparation of DNA-based graphene materials easy and possible for numerous bio-applications. Drug delivery particularly, the huge amount of attention is gained by the GO-based materials. High cellular uptake and extremely low cytotoxicity are shown by GO Nano sheets, as they did some explorations on these suitable nanocarriers for intracellular fluorescent nanoprobe and drug delivery. Various graphene-based composites were used for efficient drug delivery by using polymer grafting, for instance, patterned substrates of Nano-GO, fluorescent GO, functionalized GO nanoparticles, hyaluronic acid-decorated GO nanohybrids, and GO/hydrogel-based angiogenic.
Diagnostic and photo-thermal therapy
Other than the applications in drug delivery, GO-based materials are utilized for photo thermal therapy and diagnostic applications too. Alzheimer's disease is also diagnosed by using fluorogenic resveratrol-confined GO and hybrid GO-based plasmonic-magnetic multifunctional nanoplatforms. GO and gold nanostars were used in combination in therapeutic applications for ultra-efficient and effective photo thermal cancer therapy. Great potentials are possessed by rGO in photo thermal therapy against heat-induced controlled drug release and cancer due to their excellent photo thermal effect.
Antibacterial characteristics
Promising antibacterial characteristics with a wide antibacterial spectrum are shown by both GO and rGO finally because of their excellent antibacterial mechanism and remarkable physicochemical characteristics. These structures' antibacterial activity is enhanced by using metals like gold and silver often as nanocomposites materials with rGO and GO. ZnO/GO composites of ZnO’s varying contents in high quality and excellent antibacterial characteristics are possessed by these composites against Escherichia Coli with low cytotoxicity. Dental pathogens are killed by using the GO-based material’s antibacterial characteristics.
Conclusion
Graphene is an enthralling substance with unique optical, electrical, and thermal properties, as well as mechanical strength and other properties. Graphene is a two-dimensional sheet of carbon atoms arranged in a chicken-wire pattern. Graphene is the center of intense R&D, but its relatively expensive price is a barrier for the time being.
If you are interested, you can visit our Blografi.
References
https://apps.dtic.mil/sti/pdfs/ADA540573.pdf
https://www.researchgate.net/profile/Svetlana-Kotova-5/publication/257834884_Reduced_graphene_oxide/links/5d052319299bf12e7be2b76f/Reduced-graphene-oxide.pdf
https://www.nature.com/articles/ncomms1067.pdf?origin=ppub
https://www.graphene-info.com/reduced-graphene-oxide-introduction
https://www.graphenea.com/pages/reduced-graphene-oxide#.YapJPVVBx1t
https://www.nature.com/articles/s41598-018-28353-6
Recent Posts
-
Advanced Materials for Unmanned Aerial Vehicle (UAV) Protection Against Laser
Consider a UAV on a critical mission, rendered inoperative by a sudden laser attack. With the increa …26th Jul 2024 -
Simulation and Modeling of Material Properties
Our world is composed of a dazzling array of materials, each with its own unique properties that dic …19th Jul 2024 -
Advanced Coatings for Superior Corrosion and Wear Resistance
Corrosion and wear pose significant challenges across various industries, leading to substantial eco …12th Jul 2024