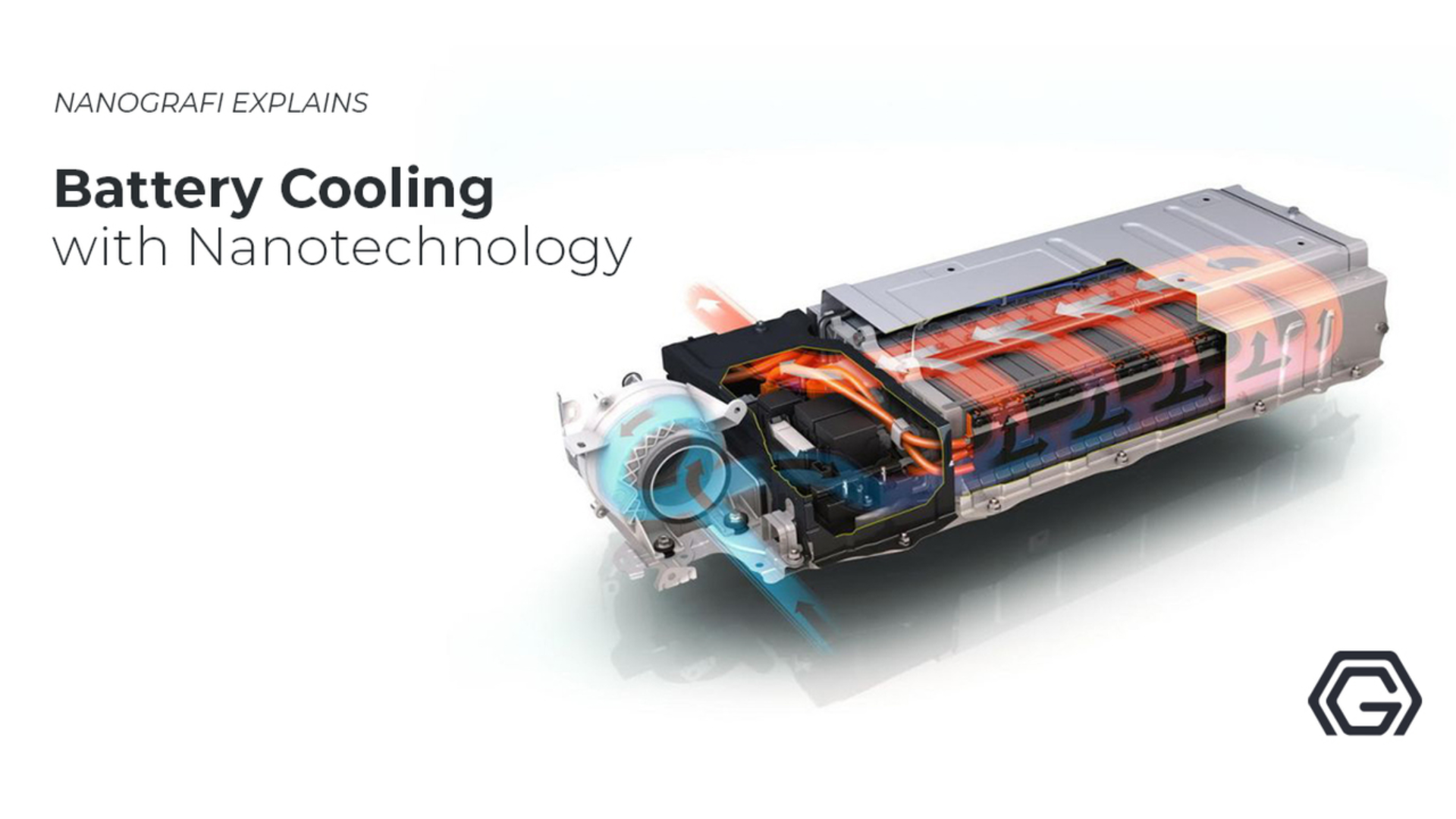
Battery Coating with Nanotechnology
Nanomaterials are those materials which are present on a nanometric scale which means it is below nm in either one of its dimension. They are known as nanomaterials because of their size and their very high fraction as well.
The PVD and CVD that are in existence are useful in processing for the preparation of microcrystalline coatings because it is very useful for the production of nanostructured coatings. For the purpose of further research in battery technology, the major drivers are to find appropriate electrodes materials with the highest possible surface area. The high surface area allows the free-flowing of charge which leads to shorter discharge/charge cycles, and higher capacity. The nanostructured materials provide a large enhancement in electrolyte material's surface area, and also the gels or ceramic’s conductivity can be enhanced by the nanoparticles sufficiently for permitting them to replace the liquid electrolytes, lessening or removing the chance of having a short circuit. We offer high-quality materials with nanostructures to significantly enhance your battery performance. Contact us to learn more about our products and optimize your battery performance.
Introduction
The nanomaterials are those materials which are present on a nano-metric scale which means it is below nm in either one of its dimension. The physical properties which the nanomaterials show are uniformity, conductivity, or the optical properties which makes these nanoparticles very much desirable in the fields of science and biology. Nanomaterials are so small that they cannot be seen an ordinary microscope and instead an electron microscope is used to view these materials. These materials occur at a great scale in nature and are often attracted to study in various fields of science such as biology, geology, physics, and chemistry. They are present at transition in between the bulk materials and atomic or molecular structures and because of this, they show a phenomenon that is not observed at any of the scales.
Nanomaterials play a great deal of role in atmospheric pollution and are happened to be a much-needed ingredient in some industrial products, for instance, paints, metals, ceramics, magnetic particles, and plastics. Nanomaterial’s natural production is carried out by various cosmological, biological, geological, and meteorological processes. The range of nanomaterial is likewise the atmospheric dust particles range as it’s not by the interplanetary dust’s mass which still at 1000 tons per year rate is falling on earth but it’s the remarkable fraction by number.
Nanostructured Materials
The nanostructured materials which are 1–100 nm in size are very well known for showcasing excellent mechanical and physical properties. It is because of the size that they hold which is equal to the size of a grain and has a very high fraction as well. According to recent searches, a very progressive amendment has been made in different scenarios to synthesize the nano-scale materials. Initially, all the researchers were merely focused on the synthesis of nanomaterials but now they are more focused on synthesizing the nanomaterials to produce useful structures from them, and because coatings having greater wear and corrosion resistance so it is very useful in this field.
Properties of Nanomaterials
The nanomaterials possess a variety of remarkable properties, both chemically and physically. The categories of chemical properties, electronic and optical are present in the form of bulks and are very distinctive of each other. When the materials are present in nano size, their properties are hard to identify as compared to the times when they are present in the larger size. This includes both the chemical and physical properties. When in the presence of certain chemicals and their combinations, the properties of these materials may vary. The basic characteristics of these materials are that they possess a definite shape with approximate fixed size, possess the surface characteristics, and also have an inner structure. The physical properties which the nanomaterials show are uniformity, conductivity, or the optical properties which makes these nanoparticles very much desirable in the fields of science and biology. Nanomaterials are also confronted as the emulsions (liquids in liquids), suspensions (solids in liquids), and aerosols (liquids or solids in the air).
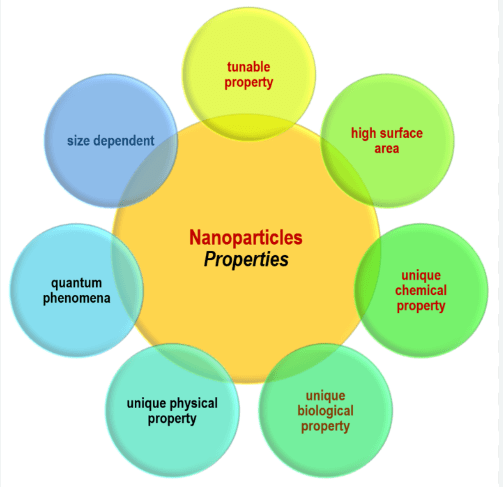
Figure 1: Unique properties of Nanoparticles.
The Importance of Battery
The devices that store electrical energy and convert it into chemical energy are known as batteries. The chemical energy gets released slowly with time when needed though. This is a reversible chemical process in the batteries that are rechargeable, which allows the re-use of battery multiple times. The batteries are the significant devices to many technological areas, for instance, medical devices, transport, power tools, portable electronics, and electricity storage that the intermittent renewable sources (tidal, solar, wind, etc.) produce. Different material layers make the battery that's why a battery can store the electrochemical energy – battery at-least consists of an electrolyte, a cathode (negative electrolyte), and an anode (positive electrode).
With a decrease in charge times and always increasing energy densities, battery technologies are improving, continuously. The materials that are used in the batteries are being tested daily, they have some physical limits, and almost all of our electric, hybrid cars, and electronic gadgets use high-performance batteries. Improvements via nanotechnology can offer these battery materials a new life lease.
Battery Coating with Nanotechnology
For further study in battery technology, the main drivers are the appropriate materials that are used as electrodes with the highest possible surface area, therefore enabling free-flowing of charge, leading to shorter cycles of discharge/charge and higher capacity. Another major concern is battery safety. In the Li-ion batteries, the dominant ones are the liquid electrolytes as they can cause cell rupture and when overheated, even combustion is possible. Right now, we need safety measures for preventing all of this from happening, for instance, expensive and complex process of manufacturing, increased size, increased space inside the battery. Now, all of these problems are solved by further research in the field of nanotechnology. A large increase in the surface area is provided by the nanostructured materials for the materials of an electrolyte. Liquid electrolytes can be replaced by the nanoparticles as they improve the gels or ceramic’s conductivity, thereby removing or lessening the short circuit’s chance.
Electrolyte Lithium Hexafluorophosphate (LiPF6)
Electrolyte Lithium Hexafluorophosphate is a crystalline powder white in color. Electrolyte possesses an important role in lithium-ion batteries (LIB) as the medium to carry lithium ions between the two electrodes of the battery. High purity and battery-grade electrolyte solutions are thus crucial for lithium-ion battery performance. The most common LIB electrolytes are derived from solutions of the salts of lithium-ion, such as LiPF6 in non-liquid solvents, such as solvent blend or alkyl carbonates with low prices and high purity.
The electrolyte solution is one mole of salt in a liter of solvent in a ratio of 4:3:3 by volume of EC + DMC + DEC. The maximum voltage is 4.5 V. The electrolyte is sealed in a container of stainless steel.
Lithium Cobalt Oxide (LiCoO2)
Lithium Cobalt Oxide (LiCoO2) is a crystalline powder, black in appearance with a specific area of 0.2~0.5m2/g and tap density over 2.8 g/cm3. The nature of Lithium Cobalt Oxide (LiCoO2) is basic with pH ranging from 9 to 11. The cell coin capacity of a cell of voltage level ranging from 3.0 V to 4.3 V is more than 151mAh/g with moisture level less than 0.1 %.
Lithium Iron Phosphate (LiFePO4)
Lithium Iron Phosphate is a powder form material used to synthesize cathodes for acid-based and Lithium-ion battery. It has a tap density of 1.132 g/cm3 and a resistance of 114.9 Ohms.cm. The materials contain 1.29 % of carbon with slightly basic nature at a pH of 8.92. The electrodes made from Lithium Iron Phosphate has a discharge efficiency of more than 97 % at the first capacity of 155.5 mAh/g.
To get more information about utilizations of nanotechnology in batteries,
you can read our blog post here.
Lithium Manganese Oxide (LiMn2O4)
This is also a powder form material for the synthesis of the cathode for batteries consisting of different metals which include Nickel, Iron, Sodium, Copper and metal impurity of less than 25 ppb with moisture content of less than 0.2 %. Lithium Manganese Oxide has a melting point of 400o C, the specific area from 0.4 ~ 1.0 m2/g, tap density ranging from 4 – 5 at standard room temperature and is insoluble in water.
Lithium Nickel Manganese Cobalt Oxide (LiNiCoMnO2)
Lithium Nickel Manganese Cobalt Oxide (LiNiCoMnO2), this physically powdered material consists of many metals in a particular proportion. These metals include Nickel, Manganese, Cobalt, Lithium, Sodium, Iron, Copper and a part of moisture or water. It has the first discharge capacity of 154.1 ~ 154.8 mAh/g at an efficiency of 87 %.
N-Methyl-2-Pyrrolidone (NMP)
Apparently, N-Methyl-2-Pyrrolidone is a colorless transparent liquid, with a molar mass of 99.13 g/mol. It possesses a melting point of -24 oC and boils at 202 oC with 1013 hPa. N-Methyl-2-Pyrrolidone has a flashpoint of 91 oC, the ignition temperature of 245 oC and vapor pressure of 0.32 hPa at standard room temperature. It is soluble in water, ether, alcohol, ester, aromatic hydrocarbons, ketone, and halogenated hydrocarbons.
Nickel Foam
Nickel foam is an exceptional anti-corrosive material with a porosity of 95 % consisting of 80 – 110 pores per inch. It is extensive on both sides of the length and width to some extent. These are used to make cathode for long term use and less maintenance required.
PVDF Binder
PVDF Binder for battery’s cathodes, which are mostly deployed in the manufacturing of lithium-ion batteries to maintain the active materials and their particles together in place and in contact with the existing collectors. It brings many benefits to the industry of Li-ion battery when it is used as a binder in the electrode formulation as well as in the separator design.
TIMCAL SUPER C45 and C65 Conductive Carbon Black
The two forms of conductive black carbon you mentioned, C45 and C65 Conductive Carbon Black, indeed have differences in their properties due to variations in the ratio of their contents. Due to difference in the ratio of the contents of the materials, it causes some differences in the properties and the resulting products as well. they have different values for volatile content, toluene extract, ash content, grit content for different micron size, moisture, density, Sulphur content, and metal elements.
TIMCAL Super P Conductive Carbon Black
It is the most graded level of conductive carbon black, ‘Super P’. it is used mainly as a stabilizer against UV, rubber reinforcement, black pigment, etc. It has a high density of 160 kg/m3.
As discussed, there are too many of the options to select from for the synthesis of the cathode for a battery. All the materials possess different characteristics and hence different characteristics can be introduced when the different materials are deployed in the construction of the cathode. The ideal characteristics for the materials to be used in the construction of the cathode for batteries are also defined and described. One may adjust according to the requirement of the battery, its voltage, and load.
For enhancing the Li-ion batteries energy density, Nanocomposite-based cathodes are designed. The battery is small enough so that it is capable of being implanted in the power of artificial retina and eye. While enhancing the performance, electric car’s safety requirements are met by the Li-ion batteries which have nanoparticle (Nanophosphate™) electrodes. Electrodes of Li-ion batteries that are prepared from nano-structured lithium titanate majorly enhances the capability of discharge/charge at the sub-freezing temperatures, and also increases the limit of upper temperature on which the battery stays safe from thermal runaway. The lifetime of Li-ion batteries and their discharge/charge rate is improved by the battery anodes that are using silicon nanoparticles for coating a titanium disilicide lattice.
Today, the best material for anode is Graphite due to its high conductivity because of which the collected electrons are readily passed in the circuit to the metal wires. But during charging, graphite is so-so at collecting ions of lithium. For holding on to a single ion of lithium, graphite's six carbon atoms are used. This weak grip restricts the electrode's ability to hold lithium and the ability of the battery to store power.
Far better can be achieved by silicon. Four ions of lithium can bind to each atom of silicon. Principally, an anode made of silicon in comparison to graphite can store ten times more energy. For tapping into that huge capacity, for decades the electrochemists have struggled.
Explore the future of lithium-ion battery applications,
you can read our blog post here.
Anodes can be easily made from silicon chunks; the difficulty is that all the anodes don't last much. The material of anode swells 300% when the battery gets charged, sending ions of lithium rushing to bind to atoms of silicon. Then, the anode shrinks again rapidly during the discharge cycle of the battery when the ions of lithium rush out. After a few such cycles, electrodes made of silicon fractures and then split into isolated, tiny grains. The battery and the anode crumble and dies.
Cui was a researcher who learned that in comparison with the materials in bulk, nanomaterials usually behave differently. For instance, in contrast with the atoms in their interior, they have a higher number of atoms on their surface, and as the lesser atomic neighbors lock the surface atoms in place, they are capable of moving in responses to strains and stresses, more easily. Atomic movement of different types demonstrates that why in comparison to the aluminum metal's chunks or can of wood, aluminum foil's thin sheets, or paper can bend more easily without breaking. Cui thought in 2008 that the nanosized silicon wires fashioning a silicon anode may release the strain and stress that reduces the anodes of bulk silicon. The thought worked. Later, Cui and his mates displayed that a little damage is suffered by the nanowires when the ions of lithium moves in and out of those nanowires of silicon. 75% of the theoretical energy storage capacity is still retained by the anode even after ten continuous discharging and charging cycles.
As compared to the bulk silicon, silicon nanowires are way more expensive and complex to be fashioned. Then the ways were being devised by the colleagues to produce less expensive anodes of silicon. Firstly, a way was found for making anodes of Li-ion battery from nanoparticles of spherical silicon. They were cheap but there was a problem. Nanoparticles are bounded together by the glue, when the atoms of lithium move out and in, the glue starts to crack leading to the nanoparticles' swelling and shrinking. A chemical reaction that coated the liquid electrolyte in a non-conductive layer is driven by that liquid electrolyte which is seeped in between the particles. This is called solid-electrolyte interphase (SEI) and it grows thick enough for disrupting the anode's capability to collect the charge. It's more of a 'scar tissue'.
Another solution of nanotech was hit by Cui and his colleagues after some years in which nanoparticles that were like an egg were created, each of its small nanoparticles of silicon was surrounded, the yolk, with a carbon shell that's highly conductive therefore letting the ions of lithium pass through. The yolk's silicon atoms had a large enough room to shrink and swell due to the carbon's shell, meanwhile also saving them from the electrolyte and an SEI later forming reactions. Cui reported in 2012 paper that after a thousand discharging and charging cycles, 74% of the capacity is retained by the anode of yolk-shell. After 2 years, they did more good by assembling the yolk-shell nanoparticles' bunches into collections of micrometer-scale resembling miniature pomegranates. The lithium storage capacity of anodes was enhanced by the silicon spheres bunching, it also lessens the electrolyte's side reactions that are not needed. It is reported that after a thousand cycles of charging and discharging, 97% of the original capacity is retained by the new material based batteries.
Conclusion
The nanostructured materials which are 1–100 nm in size are very well known for showcasing excellent mechanical and physical properties. Initially, all the researchers were merely focused on the synthesis of nanomaterials but now they are more focused on synthesizing the nanomaterials to produce useful structures from them, and because coatings having greater wear and corrosion resistance so it is very useful in this field.
To get more information, you can visit Nanografi.
References
Battery Cooling with Nanotechnology - Nanografi Nano Technology. (n.d.). Retrieved February 12, 2024, from https://nanografi.com/blog/battery-cooling-with-nanotechnology/
How to build a better battery through nanotechnology | Science | AAAS. (n.d.). Retrieved February 12, 2024, from https://www.science.org/content/article/how-build-better-battery-through-nanotechnology-rev2
Nanotechnology in Batteries. (n.d.). Retrieved February 12, 2024, from https://www.azonano.com/article.aspx?ArticleID=3042
Nanotechnology in Batteries (Nano Battery). (n.d.). Retrieved February 12, 2024, from https://www.understandingnano.com/batteries.html
Stella, B., Arpicco, S., Gil, J., Özdemir, O., & Kopac, T. (2022). Recent Progress on the Applications of Nanomaterials and Nano-Characterization Techniques in Endodontics: A Review. Materials 2022, Vol. 15, Page 5109, 15(15), 5109. https://doi.org/10.3390/MA15155109
The Future of Lithium-Ion Battery Applications - Nanografi Nano Technology. (n.d.). Retrieved February 12, 2024, from https://nanografi.com/blog/the-future-of-lithiumion-battery-applications/
Recent Posts
-
Advanced Materials for Unmanned Aerial Vehicle (UAV) Protection Against Laser
Consider a UAV on a critical mission, rendered inoperative by a sudden laser attack. With the increa …26th Jul 2024 -
Simulation and Modeling of Material Properties
Our world is composed of a dazzling array of materials, each with its own unique properties that dic …19th Jul 2024 -
Advanced Coatings for Superior Corrosion and Wear Resistance
Corrosion and wear pose significant challenges across various industries, leading to substantial eco …12th Jul 2024