Application of Boron Doped Graphene as electrode material for supercapacitors
Graphene particles are by far the best-known particles that are serving as one of the best catalyzers in the industry. When boron is doped with graphene by going through different processes it becomes and forms boron-doped grphene particles which serve various purposes in the industry.
The characteristics and properties which are exhibited by boron-doped graphene particles are highly rich in their content and are capable of exhibiting a lot of valuable processes. Technological applications have only increased over time and enhanced the entire purpose of forming these boron-doped graphene particles.
Introduction
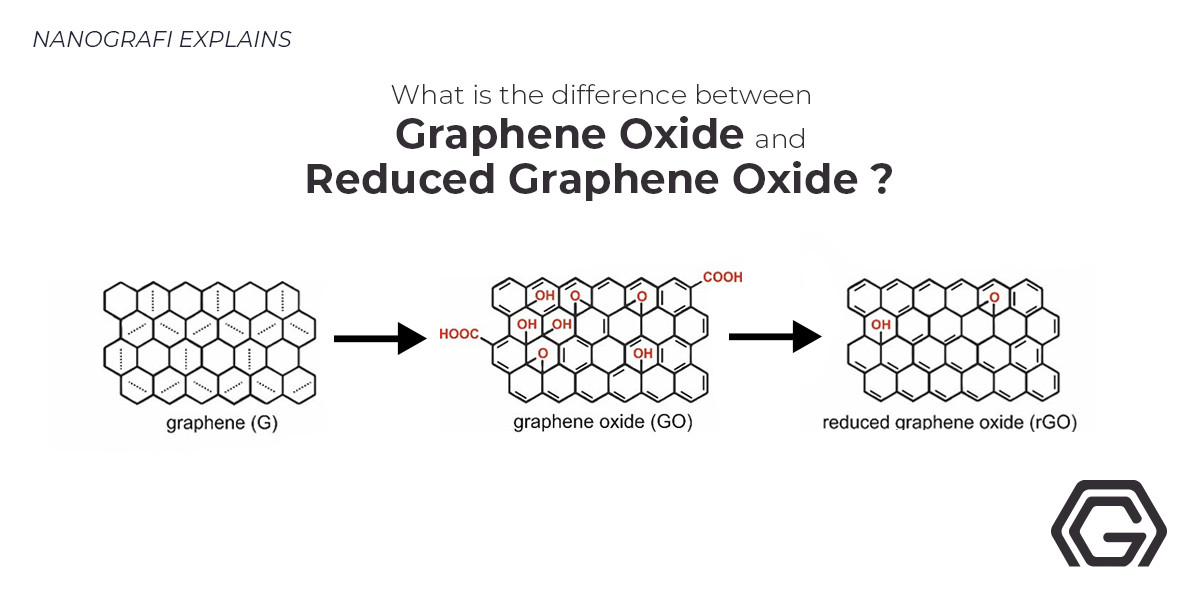
Graphene-based nanomaterial, boron-doped graphene (BG), is a carbon atom's single sheet organized in a hexagonal lattice. When the boron atom impurities are added into pure graphene, the bandgap opens, redox reactions accelerate, catalytic ability enhances, and the activation region on its surface increases. All of these alterations give various applications to it in sensors, ultracapacitors, semiconductor devices, fuel cell chemistry, and other technologies.
Graphene doping
Using electron-withdrawing (boron) or electron-donating groups (phosphorus, nitrogen), to dope graphene is significant to change graphene's electrical characteristics. Graphene sheet's electron density changes when they are doped with electron-withdrawing (electron acceptors, p-type) or electron-donating (n-type, electron donors) groups and thus also affects graphene sheet's electrochemical characteristic. Doping level plays a very important part as carrier density is very significant to tune the material's performance.
One of the other major problems is manufacturing doped materials for synthesizing scalable methods to doped material's large quantities. In this article, a technique is showed for the scalable formation and tunable doping levels by graphene oxide's exfoliation at various temperatures in the BF3 atmosphere. There are also investigations on p-doped material's electrochemical characteristics and their comparison with the ones that the literature presents.
Nitrogen and boron-doped graphene
Unusual electrocatalytic effects are displayed by nitrogen-doped graphenes toward oxygen and H2O2 reduction which have extremely significant usages for applications in fuel cells and biosensing. In comparison with graphene, large capacitance is possessed by nitrogen-doped graphenes. Similar effects were displayed by boron-doped graphene, being increased capacitance and electrocatalytic toward oxygen reduction. According to observations, it doesn't matter which atoms are doped in graphene as these characteristics are improved. Compound oxidation is easy with increased doping of materials with electron-deficient components, whereas reduction turns difficult.
These observations are contrary to the old reports on the boron-doped graphene that catalyzes the oxygen's electrochemical reduction. Cyclic voltammetry, prompt γ-ray analysis, X-ray photoelectron spectroscopy, Raman spectroscopy, and scanning electron microscopy, are used to characterize it in detail.
Preparation
Graphene oxide's thermal exfoliation was done to manufacture boron-doped graphenes in an atmosphere at 1000 °C in N2, 800 °C in N2, and with boron trifluoride diethyl etherate in N2/H2 at 1000 °C. Graphene oxide goes through many stages, exfoliation, deoxygenation (manufacturing CO2, CO, H2O, organic molecules), and then its simultaneous doping with boron. Cyclic voltammetry (CV), Raman spectroscopy, X-ray photoelectron spectroscopy (XPS), scanning electron microscopy (SEM), and prompt γ-ray analysis (PGAA), are used to characterize resulting boron-doped graphenes for studying the electrocatalytic characteristics of it towards oxygen reduction and its capacitance. Here, there are all abbreviations of all boron-doped graphenes as B-G.
Thermal shock treatment
There were investigations on the Graphene oxide's thermal shock treatment in the BF3 atmosphere and how it results in Graphene oxide's exfoliation. SEM studied boron-doped graphene's (B-G) morphology. SEM images are shown of boron-doped graphenes and those graphenes are made by exfoliation. A typical exfoliated structure is shown by all of these materials just like how it was shown in previous studies and this structure also assures graphite oxide's successful thermal exfoliation in the BF3 atmosphere. Exfoliation temperature's effect was consequently studied upon the amount of graphene used to dope boron through PGAA. (prompt γ-ray analysis)
Trace levels of elements
One of the best methods to determine the element's trace level is PGAA. Boron's absolute content in samples was determined comparatively to the liquid standard of H3BO3 with the known concentration of boron. In N2/H2 atmosphere, graphite oxide's exfoliation at 1000 °C resulted in graphene's doping at 23 ppm boron levels, whereas boron's larger amount was introduced on graphite oxide's exfoliation in the atmosphere of nitrogen, 590 ppm at 1000 °C, and 140 ppm boron at 800 °C.
According to the result, graphene can be doped with boron atoms of a higher amount if the exfoliation temperature was higher. A major decrease was observed in the concentration of boron for GO exfoliated in the atmosphere with hydrogen. Thus, boron’s successful doping into the graphene sheets is confirmed by this result.
Characterization of density
Raman spectroscopy is used by us for characterizing the defect density in boron-doped graphene by determining the doping level that boron did on Graphene. Defects are present and indicated by the D band at almost 1350 cm−1 because of the sp3 -hybridized carbon atoms. A G band is shown by the graphene sheet's pristine sp2 lattice carbon atoms at almost 1560 cm−1. The carbon structure's degree of the disorder can be indicated through the usage of the ratio between D and G band intensities (ID/IG). 0.732, 0.632, and 0.903 are the ID/IG ratio for 590 ppm B-G, 140 ppm B-G, and 23 ppm B-G, respectively.
Sizes of crystallites
D- and G- band intensities can be used to estimate various material's average crystallite sizes (La) by applying this equation, 1.20 =× × × λ − L II 2.4 10 / a 10 laser 4 G D. In this equation, ID and IG are Raman D and G band's intensities, and the excitation laser's wavelength is denoted by λlaser in nanometers. The crystallite sizes in graphenes that are doped with boron are then calculated. La is 23.0 nm of 590 ppm B-G, 26.6 nm of 140 ppm B-G, and 18.6 nm of 23 ppm B-G. At 2700 and 1620 cm-1, both the 2D band and D' band are displayed by all three boron-doped graphenes indicating disorder of low degree in their carbon structure.
XPS was used to consequently determine boron-doped graphene's elemental compositions as it is a chemical analysis method and is beneficial for determining the bonding arrangement and the elemental composition. The obtained high-resolution XPS of C 1s and wide scan of C 1s for three graphenes referred to the fact that there was a removal of most of the oxygen-containing groups and the preparation of the thermally reduced graphenes was successfully done.
To get more information,
you can read our blog post here.
Obtained ratios
According to wide scan XPS, for 590 ppm B-G, the C/O ratio is 16.1, for 140 ppm B-G, the C/O ratio is 8.9, and for 23 ppm B-G, 14.2 is the C/O ratio. Huge insight is given of the residual oxygen-containing group's chemical composition by high-resolution XPS of the C 1s signal. They exhibit different and various energy levels. When made at 800 °C in N2, 1000 °C in N2/H2, and 1000 °C in N2, boron-doped graphenes possess C-C bonds, C−C bonds, C−O bonds, CO bonds, and O−CO bonds, respectively. XPS can determine the residual oxygen-containing groups in the reduced graphene. However, trace amounts of boron can't be measured by XPS as it is not sensitive enough.
Combustible elemental analysis
Boron-doped graphene's elemental composition can be determined by using combustible elemental analysis as it is very beneficial for measuring the accurate content of combustible elements present in the bulk material. Further, more insight regarding the bonding character and composition of graphene sheets is provided when it is combined with XPS. 5.54 atom % of O, 9.63 atom % of H, and 84.83 atom % of C, was contained by 590 ppm B-G. 7.69 atom % of O, 10.03 atom % of H, and 82.28 atom % of C were contained by 140 ppm B-G. 7.48 atom % of O, 10.04 atom % of H, and 82.48 atom % of C were contained by 23 ppm B-G. Moreover, there was no nitrogen in any of the samples.
Electrical resistivity
Four-point measurements of compressed tablets studied resulting boron-doped material's electrical resistivity. 13 ppm B-G, 140 ppm B-G, and 590 ppm B-G, has the electrical resistivity of 9.5 × 10−5 Ωcm, 2.1 × 10−5 Ω cm, and 2.3 × 10−4 Ωcm. According to these results, the electrical resistivity is increased by boron's higher content which is in accordance with boron's electron acceptor characteristics. There was an investigation on the boron-doped graphene's electrocatalytic activity toward oxygen reduction reaction (ORR). In both N2 saturated 0.1 M KOH aqueous solution and air, cyclic voltammograms were recorded at 3 of the boron-doped surfaces. A major reduction peak is observed. There was no electrochemical reduction in N2- saturated KOH solution, and the reduction peak for air-saturated KOH solution was ∼−460 mV.
Cyclic voltammograms
Oxygen’s reduction originates ∼−460 mV reduction peak. Reduction of oxygen with different levels of doping was recorded by Cyclic voltammograms. For Graphenes with boron dopant's increasing concentrations, reduction peak shifts to increasingly negative potentials. Thus, for boron-doped graphene, the electrocatalytic activity towards the reduction of oxygen would be lower if the doped boron atoms are more, and vice versa. This is in agreement with hole-doping electrochemistry's general concept but also in contrast to the past observations.
Weight specific capacitance
Cyclic voltammetry capacitive charge-discharge current was used to determine the boron-doped graphene's weight-specific capacitance. ν C = I (2), is used to express the capacitance. In this equation, ν is the scan rate (mV/s, can be expressed as dE/dt), I is current (A), and C is capacitance (F). There were investigations on the capacitances of boron-doped graphenes. The investigations were being done by measuring the current With scan rates of 400, 200, 100, 75, 50, and 25 mV/s and at 0.15 V of applied potential. Boron-doped graphene's weight-specific capacitances are 10.3, 10.7, and 11.7 at 1000 °C in N2, 800 °C in N2, and at 1000 °C in N2/H2. These weight-specific capacitances refer to the fact that when the amount of doped boron atoms increases, the weight-specific capacitance decreases.
In comparison to non-doped parts
As compared to its non-doped counterparts, N-doped graphenes and B-doped graphenes exhibit higher capacitance contrary to the previous claims. No theoretical background explanation is present here whereas, in both hole and electron doping cases, improved capacitance should be expected. In Han et al.'s paper, the utilized method was based on a famous hydroboration reaction from organic chemistry. With the BH2 group being covalently bonded to the backbone of graphene, the production of sp3-hybridized carbon can be imagined from such a reaction's mechanism. Attaining carbon's direct substitution with boron through the methods that Han et al. presented, is impossible.
Surface plasmon resonance
Optical sensors, surface plasmon resonance (SPR) biosensors, gained a lot of attention because of their benefits like non-invasive measurements, room temperature real-time measurements, and high sensitivity. At the metal-dielectric interface, SPR is very sensitive to refractive index alteration. Because of the analyte's presence, sensitivity in detection is exploited if there is any kind of change at the interface. Typically in SPR biosensors, gold is utilized as a resonant layer because of its chemical stability, where a higher shift of resonant angle with a change in refractive index is exhibited by gold. Although, biomolecule’s adsorption or detection at low concentrations is extremely difficult or even impossible at times because of gold’s inertness.
Single crystalline gold surface
At increased temperatures and pressures or under high vacuumed (UHV) conditions, the single-crystalline gold surface doesn’t specifically measure small molecules adsorption. Such molecules are NH3, NO, O2, H2, and CO. There have been investigations of the physically and chemically modified gold layer in various studies regarding improved detection and adsorption mechanisms, like CNTs, gold nanocrystal, kinked/stepped gold surface gold nanorods, and graphene oxide coupled with gold nanoparticles.
Novel materials
There have been investigations on some of the novel materials like MOS2, Au/Ag/Au/chitosan-graphene oxide (RGO), and graphene, for improving SPR sensor's selectivity and sensitivity. In all of these novel materials, the most promising material is graphene because of its excellent and remarkable characteristics like high surface-to-volume ratio and high mobility that are advantageous for biomolecule’s effective adsorption in comparison with gold. An improved SPR response is displayed by the doped graphene oxide (GO) that is enriched on the gold layer's top with abundant defect sites. Although, chemical doping causes the modulation of the optical bandgap and GO’s Fermi energy which leads to alteration in the optical characteristics like dielectric constants and refractive index. SPR sensing techniques can monitor these variations.
Enhancements in graphene’s characteristics
According to recent literature, huge enhancements have been seen in the characteristics of graphene by doping graphene with various heteroatoms like Boron (B), Nitrogen (N), Oxygen (O)< and Fluorine (F). In numerous applications like improved photogenerated catalysis, solar cells, Li-ion batteries, and supercapacitors, there have been reports of the boron-doped graphene oxide. Also, numerous methods like chemical vapor deposition (CVD), arc-discharge process, and hydrothermal method have been used to dope graphene-based materials, where for tuning graphene's electronic and optical characteristics, stable atomic substitution with carbon is extremely required. At high temperatures, GO is mostly lessened thermally among the reduction methods. Although, high-density defects are produced by reduction at high temperatures on the basal planes and edge, deteriorating graphene’s optical and electrical characteristics.
Catalyst deposition
In post-treatments, there were decorations of each of the as-synthesized materials with platinum catalyst nanoparticles of 60 wt % that were made in the already reported procedure by utilizing a metal-organic Pt (acac) 2 precursor. The powdered support materials were briefly mixed uniformly in a glass vial of 4.5 mL with Pt(acac)2 in suitable ratios for yielding 60 wt% Pt. The mixture was heated under a low vacuum of 600 Torr for 16 hours at 210 C in an N2-H2O atmosphere, resulting in Pt metal nanoparticle's deposition onto the support.
Cell fabrication
An anode-supported configuration was used to fabricate solid acid fuel cells. Mesh discs of 2.85 cm2 of Sintered 304 stainless steel functioned as the anode current collectors. There have been some previous reports on the architecture of the cell and after this, it was confirmed as the architecture of this cell was completely identical to the one in previous reports. CDP’s mechanical mixture formed identical anodes with 1.0 mg/cm2 (Pt) Pt loading. For all of the tested cells, those identical anodes with a Pt loading of 1.0 mg/cm2 (Pt), inhouse synthesized 60 wt% Pt supported on naphthalene (a fugitive binder), and carbon black (Cabot Crop., Vulcan XC-72R), by weight, in a 3:1:1 ratio.
A CDP membrane was possessed by each cell. The membrane's thickness was 50 μm, and it was laminated at 125 MPa to the anode. Experimental cathodes of 25 mg of a 3:1 by CDP’s weight mixture and 60 wt% of Pt supported on CB9Ar, CH5Ar, or SWCNHs were implemented at 30 MPa pressure to the dense CDP membrane. 1.3 mg/cm2 was the experimental cathode’s areal Pt loading.
To get more information about graphene and graphene products,
you can read our blog post here.
Electrochemical testing
At 250 C temperature, ultrahigh purity H2 and the air was used to conduct cell testing by utilizing stainless steel test rigs, each at 75 C dew point. Bio-Logic VSP potentiostat was used to record polarization curves from open circuit potential to 0 V by scanning the cell potential at 10 mV s-1. At 0.8 V, each cell faced a multi-step cyclic testing protocol which included potentiostatic electrochemical impedance spectroscopy (EIS) spectrum for attaining the average high-frequency area-specific resistance (ASR), polarization curve’s recording, at 0.6 V a 30-minute potentiostatic hold, and then this protocol repeated. At 0.6 V, the cells were held after 30 cycles.
The polarization curves were corrected by using ASR for effects of membrane ohmic resistance, yielding iR-free polarization curves. For the formulation of each cathode, the results were averaged across multiple cells comprising two replicates at least.
Characterization methods
There were performances of electron energy-loss spectroscopy (EELS) and transmission electron microscopy (TEM) imaging in a Zeiss Libra 200MC (0.1 nm is the information limit). ImageJ software was used to make an estimate of the dimensions of the nanoparticle from the TEM micrographs for calculating their cross-sectional areas and for tracing their parameters. There were derivations of the same area's effective nanoparticle diameters. There have been many reports on the standard deviations and mean dimensions.
Sizes of the flake were measured by using a similar procedure, but by utilizing the square approximation. The concentration of the boron was determined by using EELS, and the background spectra were fit by utilizing the combination of a polynomial model and a power law. A polynomial model is, E−r + aE2 + bE + c, where E is energy, whereas r, c, b, and a, are fitting parameters. An In-lens detector was used to perform scanning electron microscopy (SEM) imaging at 3kV with a Zeiss Auriga 40. Pore size analyzer and Quantachrome Micromeritics surface area were used to perform BET surface area analyses. At 10 C/min ramp rate in air, Q50 from TA instruments was used to perform thermogravimetric Analysis (TGA).
Moreover, a continuous scan was done to carry out X-ray diffraction (XRD) measurements on a two-circle high-resolution X-ray powder diffractometer through the usage of Cu-Kα1 radiation (1.54059Å) with ½° fixed divergence slit.
Raman spectroscopy
A custom microscope-based system was used to perform Raman spectroscopy at 532 nm of excitation wavelength. The laser beam was focused by using a 100X objective with ~1 μm spot size on the sample, and ~0.5 mW was the measured power of the laser at the sample location. Spectral peak fitting was applied along with Gaussian/Lorentzian line shapes.
Conclusion
A lot of detailed processes are carried out to form these boron-doped graphene particles which are not only serving in the technological industries but are also paving way for the upcoming technologies which can be buckled up by the use of boron-doped graphene particles. Hence, these are excellent means of promoting technology and technology-based industries.
To get more information, you can visit our Blografi.
References:
http://utw10193.utweb.utexas.edu/Archive/RuoffsPDFs/343.pdf
https://pubs.rsc.org/en/content/articlehtml/2017/ra/c6ra25457h
https://www.sciencedirect.com/science/article/abs/pii/S0013468613012942
https://www.sciencedirect.com/science/article/abs/pii/S0379677916302351
https://www.sciencedirect.com/science/article/abs/pii/S0008622314009555
https://www.sciencedirect.com/science/article/abs/pii/S0169433218334913
Recent Posts
-
Advanced Materials for Unmanned Aerial Vehicle (UAV) Protection Against Laser
Consider a UAV on a critical mission, rendered inoperative by a sudden laser attack. With the increa …26th Jul 2024 -
Simulation and Modeling of Material Properties
Our world is composed of a dazzling array of materials, each with its own unique properties that dic …19th Jul 2024 -
Advanced Coatings for Superior Corrosion and Wear Resistance
Corrosion and wear pose significant challenges across various industries, leading to substantial eco …12th Jul 2024