Anti-Corrosive Nanocoatings
Anti-corrosive nanocoatings offer fine solutions to the corrosion related risks and failures. The unique properties of nanoscale systems and nanoparticles provide enhanced corrosion prevention, resistance, and lighter application.
Anti-corrosive nanocoatings can be metallic, ceramic, or composite. These coatings are utilized on machinery, everyday items, building materials, spacecraft, automotive, and aircraft parts. For those seeking state-of-the-art materials, Nanografi provides an extensive range of advanced materials designed to meet the highest standards of corrosion resistance and durability. Discover more about Nanografi's innovative solutions and how they can enhance your products and projects
Introduction
Corrosion is one of the most researched phenomena in materials sciences since it is a crucial problem causing degradation, failure, serious accidents, and hazards in many industrial processes and domestic systems. It is defined as the deterioration of a material (usually a metal) by the corrosive elements in its environment such as oxygen, chlorine, fluorine, or carbon dioxide. As a result of this interaction between the metal and its surroundings; corrosion products, cracks, or pits are formed on the material. Despite the popular opinion rust is not the only result of corrosion rather it is a specific corrosion product related to iron and iron based materials.
In fact, corrosion products formed on aluminum and zinc are white while the corrosion product of nickel is green, and the corrosion product of cobalt is pink. On the other hand, sometimes no corrosion product is observed and corrosion only causes cracks and pits in the structure of the metal. Corrosion damages have economic effects such as repair and maintenance costs, loss of materials, damage to equipment, a decrease in efficiency, and loss of useful or productive life. In addition to economic effects, there are also social effects of corrosion including safety impacts (fire, explosions, the release of toxic products), health impacts (personal injuries, pollution due to contamination of toxic products), the depletion of resources, etc. The global cost of corrosion is estimated to be $255 billion USD. These economic and social effects show that anti-corrosive studies have merits.
The Role of Nanocoatings in Corrosion Prevention
There are three different approaches to anti-corrosive solutions;
- material selection,
- adjusting the environmental conditions,
- cathodic production, and surface modifications.
Coating, which is one of the surface modification methods, is the most widely used method for preventing, minimizing, or controlling corrosion. The wide range of coating materials and processes for different applications and conditions make this method a desirable approach to corrosion prevention. In general, coatings reduce corrosion by providing passive or active protection. Passive protection is obtained when the coating forms a physical barrier of oxides between the substrate and the surrounding environment. Active protection is obtained when chemicals (inhibitors) are added to aggressive environments to prevent corrosion. Inhibitors minimize the corrosion rate by either being chemically absorbed on the surface of the material and forming a protective film or by reacting with the corrosive component. Traditional coating materials include polymer based coatings, chromium based coatings, zirconium based nanocoatings, etc. However, anti-corrosive materials need further development to provide better protection and meet the requirements of developing technology. This is where nanotechnology comes into play.
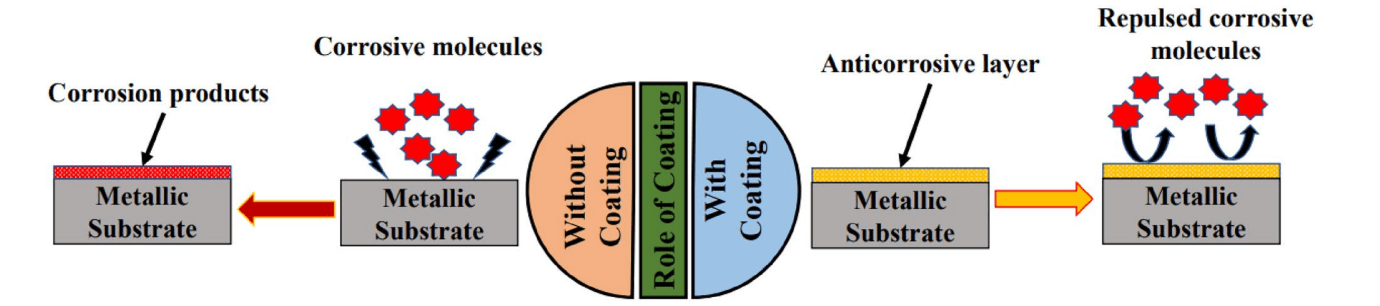
Figure 1. The role of corrosion protection.
Nanotechnology is a great asset for providing better anti-corrosive properties as well as overcoming the drawbacks of traditional coatings. For example, polymer coatings require multiple layers while chromium based coatings are notorious for being toxic. The desirable properties of nanocoatings stem from the fine size of particles, high surface areas, and different behavior of materials at the nanoscale.
-The fine size of particles in nanocoatings enables filling and blocking of the spaces on the metals surface preventing corrosive materials to diffuse.
-The high surface area provides better adhesion properties, which improve the lifetime of the coating. Furthermore, nanosized materials provide better mechanical, chemical, and electronic properties making nanocoatings stronger and harder, more resistant to corrosion and wear.
For example, while microscale zinc coatings show poor weldability, zinc nanocoatings overcome this problem. It is also possible to develop multifunctional anti-corrosive nanocoatings with self-healing, self-cleaning, corrosion sensing, and wear resistance. Additionally, nanotechnology is utilized to develop smart anti-corrosive coatings that respond to external stimuli such as pH, humidity, heat, stress, coating distortion, electromagnetic radiation, etc., by releasing controlled amounts of inhibitors in order to repair and cure defects and damages.
The desirable properties of anti-corrosive nanocoatings are utilized in several different areas. Products that are daily used by customers such as electronic devices, eyeglasses, etc. are all coated with anti-corrosive layers. In the industry, construction materials such as support scaffolds, windows, air filters, etc. as well as industrial machinery and automotive parts utilize anti-corrosive coatings. Furthermore, metallic biomaterials used in medical applications such as bone fixation materials and implants require bio-functional nanocoatings. Anti-corrosive nanocoatings are also utilized for the protection of wood materials.
Production of Anti-Corrosive Nanocoatings
There are several different production methods for anti-corrosive nanocoatings. These methods are mainly divided into three categories;
- mechanical,
- physical, and
- chemical deposition.
The mechanical deposition is the cheapest hence the most widely utilized deposition technique and can be achieved through spray, paint, spin-coating, or dip-coating. Physical deposition can be done by either bonding, condensation, or sputtering. Physical bonding techniques include physical diffusion bonding which is carried out under moderate temperature and pressures, brazing bonding which is carried out under high temperatures, surface-activated bonding (SAB), and selective laser sintering which is a novel 3D printing technique. Condensation techniques include physical vapor deposition (PVD) which is a particularly popular deposition technique under vacuum and liquid phase epitaxy (LPE) which is performed at regular pressure conditions.
Sputtering is another popular deposition technique since it provides precise epitaxial growth with strong bonds. However, it is more expensive compared to bonding and condensation techniques. Different sputtering methods include molecular beam epitaxy (MBE), radiofrequency magnetron, and pulsed laser deposition (PLD). Chemical deposition techniques are usually cheaper but usually involve expensive precursors. Hence these techniques are commonly preferred for small scale applications. Chemical deposition techniques include the famous sol-gel technique, Langmuir, atomic layer deposition, and plating. Each of these techniques has its own advantages and disadvantages. For example, pulsed laser deposition can produce dense and uniform coatings; however, they suffer from thermal expansion. The appropriate coating method must be chosen according to the substrate material and the application area. The optimum coating technique must provide uniformity, smoothness, adhesion, crack free surfaces, and minimized agglomeration of nanoparticles.
What are the Most Important Anti-Corrosive Nanocoatings?
With the increasing demand for anti-corrosive nanocoatings, researchers have developed several different alternatives with different purposes and corrosion properties. The anti-corrosive nanocoatings can be mainly divided into three groups; metallic nanocoatings, ceramic nanocoatings, and nanocomposite coatings. Ceramic nanocoatings include titanium oxide, tantalum oxide, alumina, zirconia, and graphene based nanocoatings. Nanocomposite coatings can include metallic or polymeric matrices harboring several different nanomaterials.
Metallic Nanocoatings
Metals such as cadmium (Cd), Nickel (Ni), Tungsten (W), Zinc (Zn), Phosphorous (P), Cobalt (Co), Iron (Fe), and Copper (Cu) are utilized as metallic anti-corrosive nanocoatings. Metallic nanocoatings can be made up of pure metals or alloys of metals. These nanocoatings utilize the different behavior of metallic materials at the nanoscale and the excellent charge distribution of metals. The deposition of metallic nanocoatings can be achieved through more than one method. The most frequently used deposition methods for metallic nanocoatings are sputtering, multi-arc ion plating, and electrodeposition.
The anti-corrosive behavior of metallic nanocoatings is strongly related to various factors including composition, coating dimensions, grain size, the coating method, additives, pH of the environment, and surface morphology of nanocoating. Nickel alloys such as Ni-W, Ni-Zn, and Ni-P have attracted considerable interest as metallic anti-corrosive coatings. In addition, cobalt and cobalt alloys have been considered as a promising replacement for toxic chromium coatings. The corrosion resistance of alloys is affected by the alloy composition significantly. For example, 13.31 wt.% (26-nm grain size) and 17.62 wt.% (37-nm grain) of Ni content show the best corrosion resistance for Ni-Zn alloys.
Ceramic Nanocoatings
Titanium dioxide (TiO2) is a ceramic material utilized in photovoltaics, sensors, construction material, etc. due to its unique properties. TiO2 shows ultraviolet (UV) resistance, high refractive index, photocatalytic activity, and high abrasive and corrosion resistance. Hence it is a desirable material in anti-corrosive nanocoatings. These coatings were prepared by several different coating methods including sol-gel and atomic layer deposition. Studies show that TiO2 nanocoatings deposited by the sol-gel method on stainless steel improved corrosion resistance of the material by nearly 10 times in NaCl solutions. TiO2 nanocoatings prepared by atomic layer deposition provided corrosion resistance without any cracks or pinholes. Additionally, nitrogen doped titania nanocoatings have shown enhanced corrosion resistance.
Alumina nanocoatings also show desirable mechanical properties and corrosion resistance. Hence alumina thin films are utilized in gas diffusion barriers, surface passivation applications, etc. Studies on alumina nanocoatings indicate that the sol-gel method and plasma enhanced atomic layer deposition are appropriate techniques for the deposition of alumina. Plasma enhanced atomic layer deposition enables better film density than thermal atomic layer deposition techniques. Nanostructured sol-gel alumina coatings provided better corrosion resistance in the NaCl environment. On the other hand, plasma enhanced atomic layer deposition alumina coatings require more than 10 nm thickness to effectively provide corrosion resistance.
Tantalum oxide nanocoatings are another attractive option for corrosion resistance due to their s high dielectric strength, high hardness, and high chemical attack resistance under severe conditions. Tantalum oxide nanocoatings are utilized in several different areas including microelectronics, capacitors, nanosensors, anti-reflective layers, and optical waveguides. Tantalum oxide provides corrosion resistance by forming a stable passive oxide layer on the surface in NaCl environments. Depending on the substrate and deposition method, the corrosion rate can be reduced by a factor of four with the use of tantalum oxide nanocoatings. Another tantalum based anti-corrosive nanocoating material is tantalum nitrate (T2N) which exhibits a decrease in the corrosion resistance with the increase in acidity and temperature.
Zirconia (ZrO2) is a ceramic material exhibiting desirable physical and chemical properties such as low friction coefficient, high melting point, high chemical stability, high refractive index, and dielectric constant. It is widely implemented as a coating material due to its high corrosion resistance, long wear life, and high-temperature resistance. In addition to corrosion prevention, zirconia is also utilized as thermal barrier coatings and in optic devices, magnetic storage media, catalyst support, etc. Zirconia nanocoatings are considered as a suitable replacement for chromium based anti-corrosive coatings. Several different deposition methods were tested for zirconia nanocoatings including sol-gel, thermal spraying, electrodeposition, and chemical vapor deposition. However, the sol-gel method results in a higher degree of purity. Sol-gel zirconia nanocoatings provide enhanced corrosion rates under neutral and acidic environments. Other factors considered for zirconia nanocoatings are adhesion properties and the film thickness of the coating. Achieving better adhesion prolongs the life-time of the coatings.
Graphene and graphene oxide nanocoatings are considered as other alternatives for corrosion resistance. Multilayer graphene coatings provide 7 times slower corrosion rates on copper and 20 times slower corrosion rates on nickel than bare substrates. Graphene oxide nanocoatings show corrosion resistance under NaCl environments and inhibit bacterial growth.
Learn now about the latest coating technologies to increase corrosion and wear resistance.
Nanocomposite Coatings
Nanocomposite materials which include at least two immiscible phases are also utilized for anti-corrosive coatings. The main phase in nanocomposites coatings is the matrix which hosts the filler materials. The matrix material can be polymers, ceramics, or metals. Nanoparticles are dispersed in these matrices to enhance the properties of the nanocoating. Polymer materials are good corrosion inhibitors, however; they show poor wear and scratch resistance. It is also important to note that polymeric matrices show higher porosity hence are more permeable to corrosive species. Nanomaterials included in the polymer matrix commonly provide better stiffness, strength, conductivity, thermal resistance; reduced thermal expansion, solvent attack, cost, and chemical resistance. The processing method of polymer nanocomposites is important in terms of achieving homogenous filler distribution which leads to better enhancement of properties. Chemical processes such as in situ polymerization, emulsion polymerization, solution intercalation, and melt intercalation. Out of these processes in situ polymerization and solution intercalation are considered to have the best polymer nanocomposite in terms of nanoparticle distribution. Multi-walled carbon nanotubes (MWCNT), Al2O3, graphene oxide (GO), ZrO, and SiO2 are amongst the frequently utilized nanoparticles in polymer matrices such as vinyl chloride, vinyl acetate, xylan, chitosan, epoxy resins, etc. Nanocomposite coatings can reduce corrosion by the formation of the passive film on the surface, fine distribution of electrical conductivity in the polymer matrix, and oxygen reduction on the surface. MWCNT and Al2O3 both enhance the mechanical strength and corrosion resistance of the polymer matrix. GO enhance the corrosion resistance of the polymer by providing hydrophobicity.
Aside from the aforementioned polymers, conductive polymers are also used as host matrices for nanocomposite coatings since they show increased corrosion resistance. Common conductive polymers used in nanocomposite coatings are polyaniline (PANI), polythiophine, and polypyrrole. Conductive polymers are usually enhanced with TiO2, ZnO, graphene, and CaCO3. Titanium oxide provides nearly 100 times stronger corrosion resistance. While TiO2 and ZnO improve corrosion resistance due to increased surface area, diffusion resistance, and prevention of charge transportation, CaCO3 and graphene improve corrosion resistance due to their hydrophobic nature.
Due to environmental considerations with coatings containing volatile organic compouns (VOCs), waterborne polymers are also considered as promising alternative coating matrices. These polymers are mostly used in pains as a resin dispersant, however; they can also be utilized as host matrices in nanocoatings. Water-based alkyds coatings are considered as the cheapest option for a VOC alternative. Incorporating nanoparticles such as Fe3O4, Fe2O3, and ZnO reduces the corrosion rate while providing UV protection, scratch resistance, and abrasion resistance as well.
Nanocomposite coatings of nanocrystalline metal matrices and various nanoparticles are also utilized as nanoengineered anti-corrosive coatings. Nickel alloys such as Ni-W and Ni-Co are commonly used as metal host matrices. The most frequently used nanoparticles in metal matrices are titanium dioxide, alumina, and silicon carbide. SiC and TiO2 incorporated into metal matrices provide a passivation layer on the surface of the nanocomposite coating. Thus, prevent the formation and development of corrosion products and cracks. The anti-corrosion effect of alumina in nanocomposite coatings is strongly related to the deposition method and concentration. However, optimized alumina incorporated nanocomposite coatings provide a considerable anti-corrosive effect. In addition to nickel alloys, metal nitrate nanocoatings are also widely researched for anti-corrosive applications. Both binary and ternary nitrate nanocoatings are utilized as corrosion resistant coatings. However, ternary metal nitrate coatings can operate at higher temperatures up to 900°C. These nanocoatings are often composed of Cr, Al, Ti, or Zr. Metal nitrate nanocoatings show strong oxidation resistance, hence anti-corrosive properties, and high wear resistance. On the other hand, it is important to note that Cr-based nitrates provide better oxidation resistance than Ti-based nitrates even though Cr has a toxic effect.
Multifunctional Anti-Corrosive Nanocoatings
Anti-corrosive nanocoatings are suitable canvases to include multifunctional and smart properties such as stimuli responsiveness, self-healing, self-cleaning, and corrosion sensing.
Corrosion sensing anti-corrosive nanocoatings are mostly pH sensitive coatings that contain color changing compounds sensitive to increased pH levels caused by oxidative corrosion reactions. The change in pH level also triggers the release of anti-corrosive materials. Compounds such as Schiff bases, hydroxyquinolines, fluorescein, phenolphthalein, bromothymol blue, 7-amino-4-methyl coumarin, 7-diethylamino-4-methyl coumarin, etc. are utilized in corrosion sensing nanocoatings. Self-healing properties minimize the effect of possible by repairing the possible corrosion damages in the coating structure. Nanoparticles are added to polymer coating matrices as inhibitor particles to achieve intrinsic or extrinsic self-healing effects. Active agents such as free radicals, aromatics, −OH, −Si−O, −C=C−, −COOH, −NH2, −SH, −S−S−, −C=O, etc in nanoscale. Self-cleaning is an extremely desirable property for coatings. These multifunctional nanocoatings utilize hydrophilic or hydrophobic approaches for the efficient removal of dirt. The most commonly used nanomaterials for self-cleaning anti-corrosive nanocoatings are nano-silver and nano-titanium oxide. Nano-silver provides anti-bacterial properties to the coating while nano-TiO2 provides hydrophobic and photocatalytic effects.
Conclusion
Corrosion prevention is a long standing concern of the engineering and production industry. Inhibiting corrosion reactions prolong the lifetime of machinery, everyday items, buildings, materials used in spacecrafts, automotives, and aircrafts. For over 200 years different coating materials such as varnishes and paints have been used as anti-corrosive layers. However, with the development of technology, finer anti-corrosive systems were required. At this point, nanotechnology is utilized to achieve better anti-corrosive coatings. The unique properties of nanomaterials provide better corrosion resistance with much thinner coatings.
The most commonly utilized anti-corrosive nanocoatings are grouped into three categories; metallic nanocoatings, ceramic nanocoatings, and nanocomposite coatings. Metallic nanocoatings commonly involve nickel, cobalt, chromium, tungsten, zinc, phosphorus, and iron while ceramic nanocoatings include titanium oxide, tantalum oxide, alumina, zirconia, and graphene based nanocoatings. Nanocomposite coatings can include metallic or polymeric matrices harboring several different nanomaterials. The deposition of anti-corrosive nanocoatings can be achieved through mechanical, physical, or chemical methods. The choice of deposition method is the most important factor affecting the corrosion resistance properties of the coating. In addition to the deposition method, the grain size of nanomaterials,composition, coating dimensions, additives, pH of the environment, and surface morphology also affect the anti-corrosive behavior of the nanocoatings. It is also possible to achieve multifunctional anti-corrosive nanocoatings. The inclusion of different nanoparticles to the coatings provide self-cleaning, self-healing, and corrosion sensing properties to the anti-corrosive nanocoatings. Anti-corrosive nanocoatings coatings are commonly utilized to provide corrosion resistance to metallic surfaces. However, they are utilized on wood, glass, or ceramics as well. Even though anti-corrosive nanocoatings have come a long way and are being used in several different applications, there is still room for growth and further development in this area.
To follow the latest developments and innovations related to nanotechnology, visit Blografi.
References
Abdeen, D. H., El Hachach, M., Koc, M., & Atieh, M. A. (2019). A review on the corrosion behaviour of nanocoatings on metallic substrates. Materials, 12(2), 210.
Chemical Vapor Deposition CVD Graphene - Nanografi Nano Technology. (n.d.). Retrieved July 17, 2024, from https://nanografi.com/blog/chemical-vapor-deposition-cvd-graphene/
Groysman, A. (2010). Corrosion mechanism and corrosion factors. In Corrosion for everybody (pp. 1-51). Springer, Dordrecht
Krishnamoorthy, K., Jeyasubramanian, K., Premanathan, M., Subbiah, G., Shin, H. S., & Kim, S. J. (2014). Graphene oxide nanopaint. Carbon, 72, 328-337.
Titanium Oxide Nanopowder/Nanoparticles Specifications - Nanografi Nano Technology. (n.d.). Retrieved July 17, 2024, from https://nanografi.com/blog/titanium-oxide-nanopowdernanoparticles-specifications/
Ulaeto, S. B., Rajan, R., Pancrecious, J. K., Rajan, T. P. D., & Pai, B. C. (2017). Developments in smart anticorrosive coatings with multifunctional characteristics. Progress in Organic Coatings, 111, 294-314.
Yun, H., Li, J., Chen, H. B., & Lin, C. J. (2007). A study on the N-, S-and Cl-modified nano-TiO2 coatings for corrosion protection of stainless steel. Electrochimica Acta, 52(24), 6679-6685.
Zinc Nanoparticles - Nanografi. (n.d.). Retrieved July 17, 2024, from https://nanografi.com/nanoparticles/element-alloys-nanoparticles/zinc-nanoparticles/
Recent Posts
-
Advanced Materials for Unmanned Aerial Vehicle (UAV) Protection Against Laser
Consider a UAV on a critical mission, rendered inoperative by a sudden laser attack. With the increa …26th Jul 2024 -
Simulation and Modeling of Material Properties
Our world is composed of a dazzling array of materials, each with its own unique properties that dic …19th Jul 2024 -
Advanced Coatings for Superior Corrosion and Wear Resistance
Corrosion and wear pose significant challenges across various industries, leading to substantial eco …12th Jul 2024







