Calcium Oxide Nanoparticles
Nanotechnology has wide applications in different fields of science and industry, and current research is generally focused on nanoparticle-based materials and their possible applications. In nanomaterials universe, metal oxide nanoparticles are important materials due to the widespread applications in various aspects. Calcium oxide (CaO) is an exceptionally important industrial compound, which is used as catalyst, toxic-waste remediation agent, an additive in refractory, in paint as well as for other fundamental applications due to their interesting chemical, electrical and optical properties. Calcium oxide (CaO), commonly known as burnt lime or quicklime, is an alkaline earth metal oxide. Calcium is a Block S, Group 2, Period 4 element, while oxygen is a Block P, Period 2 element. Calcium oxide is a white, alkaline, caustic, crystalline solid at room temperature. Nanoparticles that occur naturally such as volcanic ash, soot from forest fires or the incidental by products of combustion processes are physically and chemically heterogeneous and often termed as ultrafine particles. CaO is a good example of naturally abundant earth metal oxides. CaO nanoparticles have spherical or faceted high surface area, also they are magnetic nanostructured particles.

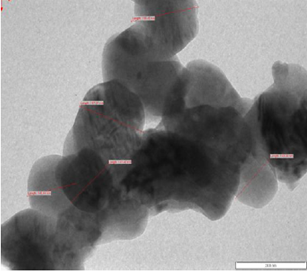
Figure 1: SEM (left) and TEM (right) images of calcium oxide nanoparticles[1].
Application in various fields like catalysis, optoelectronics, sensors and environmental remediation made metal oxide nanoparticles substantial agents in production. Calcium oxide (CaO) is one of the promising metal oxides. Calcium oxide is a high-volume chemical compound and finds itself for applications in numerous industries. In addition, calcium oxide is plenty in nature, inexpensive and easy to produce. For an industrial application example, compounds and substances such as citric acid, glucose and certain dyes are purified using calcium oxide, before further refinement. Moreover, CaO is used for balancing out acidic soil and is used in areas where rainfall washes the calcium from the soil. Also, calcium oxide finds its part in electronics as a desiccant in light emitting diodes (LED). Also, an example to calcium oxide’s applications, drugs have grown beyond therapeutic agents to growth factors and have turned more quantized. Calcium oxide nanoparticles with its nano structures are very feasible to the applications in drug delivery systems. It is also used industrially as a dehydrating agent in the creation of steel, an absorbent, as a water softener, as a potential hydrogen regulator for waste-water and in fertilizers. The properties and applications of nanoparticles depends on their size and morphology. Therefore, controlled production of CaO nanoparticles is very important for effective and successful applications. The effects on the size, shape, uniformity and properties of the nanoparticles highly depend on the temperature and nature of solvents. It is considered that solution-phase methods provide a large degree of control over the synthesized nanoparticles. Furthermore, organic solvents and high temperature have significant effects on the shape, size, uniformity and other properties of produced nanoparticles. Although the increasing temperature led to smaller particles and higher symmetry of nanoparticles but using of organic solvent as a medium of reaction involves several peptizations because of agglomeration of the synthesized particles. Hence, Cao nanoparticles can be prepared by various techniques such as sol-gel method, precipitation, hydrogen plasma-metal reaction, sonochemical synthesis, thermal-decomposition method etc.
Synthesis of CaO Nanoparticles
CaO nanoparticles are synthesized by several methods such as sonochemical, hydrogen plasma-metal reaction, sol–gel method, precipitation method and water-in-oil (W/O) micro-emulsions and also green eco-friendly synthesis of CaO NPs. Most of the methods suffer from limitations like the use of organic solvents, high temperature, long process times and complicated process equipment. In this review, some of these synthesis methods will be mentioned and discussed.
Thermal Treatment of Ca(OH)2
CaO nanoparticles are often produced via thermal treatment of Ca(OH)2 or CaCO3 as precursors. Initially, calcium hydroxide (Ca(OH)2) is dissolved in ethyleneglycol and stirred vigorously and then sodium hydroxide (NaOH) is added into the mixture. The solution is left to settle down for 5 hours after 10 minutes of sonication. The precipitate is filtered and obtained precipitate is repeatedly washed with deionized water for 5 times and then calcined in 100℃, subsequently. Finally, calcium oxide nanoparticles of different size are obtained by calcining at 800℃. The chemical reaction can be written as:
CaCl2 + 2NaOH = CaOH2 + 2NaCl (1)
Ca(OH)2 = CaO + H2O (2)
Thus, this method can be used as a simple, cheap and convenient way for producing calcium oxide and hydroxide nanoparticles at an industrial large scale.
Synthesis of Calcium Oxide Nanoparticles via Sol-Gel Method (Chicken Egg Shell)
Synthesis of metal oxide nanoparticle through sol-gel method has four basic consecutive processes: Preparation of homogeneous solution, the formation of ‘sol’ by hydrolysis, the formation of ‘gel’ by condensation and drying of the formed gel. In this method, a homogenous solution of metal salt, CaCl2, is produced by dissolving solid CaCO3 in dilute HCl as shown in Equation (1). Chicken eggshell is used as a precursor for this method. Waste eggshells are thoroughly washed with warm water and cleaned with deionized water. Then, the washed sample is dried at 120℃ for 2 h, ground into a powder and sieved with sieve size. For the preparation of calcium chloride (CaCl2) solution, PES is dissolved in 1 M hydrochloric acid (HCl).
CaCO3 (s) + 2HCl (aq) = CaCl2 (aq) + H2O (l) +CO2 (g) (1)
The next step is the formation of ‘sol’ by hydrolysis process. ‘Sol’ is defined as a stable dispersion of colloidal particles of precursors in a solvent due to hydrolysis reaction. During the hydrolysis process, metal hydroxide is formed. A total of 1 M sodium hydroxide (NaOH) is added slowly (drop by drop) to convert the homogeneous CaCl2 solution formed in the previous step into ‘sol’ at ambient temperature (Equation (2)). Then ‘gel’ formation by condensation is followed. The slow addition of NaOH resulted in a low rate of nucleation and encourages subsequent precipitation of Ca(OH)2 one over another forming a highly crystalline gel. The condensation reaction results in small-sized particles interconnected to each other forming a rigid and highly crystalline inorganic network within the liquid. Ca(OH)2 gel containing solution is aged overnight at ambient temperature.
CaCl2 (aq) + 2NaOH (aq) = Ca(OH)2 (s) + 2NaCl (aq) (2)
Then, filtration is followed where the filtrate is cleaned with distilled water in order to remove
adsorbed impurities in the precipitate. The synthesizing process ended by drying the gel. In this process, the solvent (liquid phase) is removed and a significant amount of shrinkage and densification is noticed. The powder is dried at 60℃ for 24 hours in an oven and calcined at 900℃ for 1 h (Equation (3)).
Ca(OH)2 (s) + Heat = CaO(s) + H2O (l) (3)
Read Also About Nanocalcites
Green Synthesis of Calcium Oxide Nanoparticles
The use of plant extracts in the synthesis of nanoparticles have proven to be cost effective and opens up a wide area in non-toxic nanoparticle synthesis. In this work, CaO nanoparticles are synthesized by using the Papaya Leaf and Green Tea extract.
1.Preparation of extract from Papaya leaves
Papaya extract is prepared from thoroughly washed papaya leaves. It is then boiled in distilled water for half an hour and filtered with filter paper and the resulting extract is cooled and used for synthesis of metal oxide nanoparticles.
2.Preparation of extract from Green Tea leaves
Green tea extract is prepared from washed green tea leaves. It is then boiled in distilled water for half an hour, filtered with filter paper and the resulting extract was cooled and used as the green tea extract solution.
3.Synthesis of calcium oxide nanoparticles using plant extracts
Calcium nitrate solution is added to papaya extract. Then, the mixture is stirred in a magnetic stirrer for about half an hour. NaOH is added dropwise while stirring till a white precipitate of calcium hydroxide is obtained. The precipitate is filtered and dried in an air oven for an hour. The content is washed repeatedly with distilled water to remove the basicity of the solution. Further, the calcination is done in the muffle furnace at 500⁰C for three hours. Similar procedure is carried out for synthesis of calcium oxide nanoparticles using Green Tea extract.
Applications
Calcium Oxide (CaO) nanoparticles have found several applications such as in catalysis, adsorption, water purification and also as antibacterial agents. CaO is a material of particular interest as it is regarded as a safe material to human beings and animals.
CaO NPs possess excellent antimicrobial potential and capability to inactivate microbial endotoxin. Due to CaO NPs unique structural and optical properties they can be used as a potential drug delivery agent, in photodynamic therapy (PDT), photo-thermal therapy (PTT), and synaphic delivery of chemotherapeutic agents. CaO NPs are safe material to human beings and animals. Due to good porosity it finds its part in storage systems and its biocompatibility earns it a good value in drug delivery and gene transfection.
The obtained results indicate that porous CaO microsphere can be used in transesterification reaction of biodiesel production from vegetable oils and it can be used as effective drug carrier in DDS due to its bioactive and biocompatible nature.
The BET results indicated that the porosity of prepared nano-particles was in the mesopore range and the particles could be used as catalysis and gas sorbent.
In a general way, it can be concluded that the formation of Copper-NPs is sensitive to the synthesis conditions. Therefore, before using a synthesis method described in the literature, one should observe the real influence of synthesis conditions such as temperature, stirring and composition of the mixture for an effective control of size distribution and particle shape. Also, it should be considered that the metallic nanoparticles are rather a new research subject in science world. Although copper nanoparticles have some outstanding properties, more research should be done in order to enhance the properties and moderate the disadvantages. A lot of researches are still being conducted to improve its properties and lower its manufacturing cost in order to implement its use in real life applications. In the coming years, this material will innovate the world of technology due to its unique characteristics.
References
1.Butt AR, Ejaz S, Baron JC, Ikram M, Ali S, CaO nanoparticles as a potential drug delivery agent for biomedical applications, Digest Journal of Nanomaterials and Biostructures, 10, 2015, 799 – 809
2.Suja Abraham, V P Sarathy, Biomedical Applications of Calcium Oxide Nanoparticles -A Spectroscopic Study, Department of Physics, Faculty of Science, Hindustan Institute of Technology and Science, 15 March 2018.
3.G. Oskam, Metal oxide nanoparticles: Synthesis, characterization and application, Journal of Sol-Gel Science and Technology, 37 (2006) 161–164.
4.Z. Tang, D. Claveau, R. Corcuff, K. Belkacemi, Preparation of nano-CaO using thermal-decomposition method, J. Arul, Materials Letters, 62 (2008) 2096–2098.
5.Roy, J. Bhattacharya, Micro-wave assisted synthesis and characterization of CaO nanoparticles, International Journal of Nanoscience, 10 (2011) 413–418
6.Islam, S.H. Teo, E.S. Chan, Y.H. Taufiq-Yap, Enhancing sorption performance of surfactant-assisted CaO nanoparticles, Journal of Applied Chemical Research, 4 (2014) 65127–65136
7.Ferraz, E.; Gamelas, J.A.F.; Coroado, J.; Monteiro, C.; Rocha, F. Eggshell waste to produce building lime: Calcium oxide reactivity, industrial, environmental and economic implications. Mater. Struct. 2018, 51, 1–14.
References for Image
1. A.S. Balaganesh, R. Sengodan, R. Ranjithkumar, B. Chandarshekar, Synthesis and Characterization of Porous Calcium Oxide Nanoparticles, International Journal of Innovative Technology and Exploring Engineering (IJITEE) ISSN: 2278-3075, Volume-8 Issue-2S December, 2018.
Recent Posts
-
Cellulose Nanocrystals (CNC) in Food Industry
Cellulose nanocrystals (CNC) are emerging as a pivotal material in the realm of food technology, her …3rd May 2024 -
Reducing the Carbon Footprint of Nanomaterials
The production of nanomaterials is vital for numerous advanced applications, from healthcare to elec …26th Apr 2024 -
Nanocomposites in Food Packaging
The utilization of nanocomposites in food packaging represents a significant advancement in the fiel …19th Apr 2024






