Application Areas of Graphene Paste
Graphene is known as a carbon allotrope in the industry as it is comprised of carbon atoms put together in the form of a lattice. Graphene is a highly necessary product in today’s world as it is serving in areas where its benefits are excessively high.
One such area is in the form of graphene paste. Due to its remarkable properties and characteristics, it is now being excessively used in the industry as graphene paste as the presence of graphene in the form of paste makes it easy for several different products to be worked upon ad allows them to be modified in ways that can be more beneficial and easy. Graphene oxide plays a huge role in the making of graphene powder.
Introduction
A carbon’s allotrope, graphene, has a single layer of atoms organized in a 2-D honeycomb lattice. The word “Graphene” is a combination of suffix‘ene’ and ‘graphite’, which is an indication that stacked graphene layers are possessed by carbon’s graphite allotrope.
Graphene sheet's each atom is joined through σ-bond to its three closest neighbors. In this bonding, the individual atom shares 1 electron to a conduction band, extending it over the complete sheet. In polycyclic aromatic hydrocarbons and carbon nanotubes, the bonding is similar to it whereas, in glassy carbon and fullerenes, the bonding is partially similar to the bonding in graphene. Being a semimetal, the uncommon electronic characteristics of graphene are because of these conduction bands and the theories for massless relativistic particles describe them best.
Charge carriers
Instead of quadratic, a linear dependence of energy on momentum is shown by the charge carriers in graphene. Bipolar conduction can be shown by the self-made field-effect transistors with graphene. Over long distances, the charge transport is ballistic, and nonlinear and large diamagnetism, along with large quantum oscillations are displayed by the material. Electricity and heat are very efficiently conducted by graphene along its plane. Light of all visible wavelengths is strongly absorbed by the material, accounting for graphite's black color. Thus, due to its extreme thinness, the graphene single sheet is almost transparent. As compared to any steel ever of the same thickness, this material is almost about a hundred times stronger.
According to scientists
For decades, there have been many theories about graphene by various scientists. For a long time, it has been made in little quantities by using pencils and some other graphite applications. In 1962, the electron microscopes originally observed it, but they were studied only when they were supported on metal surfaces. Andre Geim and Konstantin Noveselov later discovered, isolated, and characterized the material in 2004 at the University of Manchester, and in 2010, they won a Nobel Prize in Physics because of their studies on the material. The easy isolation of high-quality graphene came out to be a surprise.
Graphene’s Global market
In 2012, graphene’s global market was $9 million, and almost all of its demand was from development and research in composites, electric batteries, electronics, and semiconductors.
Recommendations of IUPAC
The name ‘graphite’ is recommended for being utilized by the International Union for Pure and Applied Chemistry (IUPAC) for 3-dimensional material, and "graphene" for the times when there is a discussion on the individual layers' characteristics, structural relations, or reactions. According to a narrow definition of free-standing or isolated graphene, the layer should be this much isolated from its environment that it still could include the layers that are transferred to silicon carbide or silicon dioxide or are suspended.
Properties of Graphene
Mechanical
0.763 mg/m2 is graphene’s density (two-dimensional). The stiffness (Young modulus) of graphene is near 150,000,000 psi (1 TPA). The intrinsic tensile strength of graphene is 130 GPa (19,000,000 psi) but for stretching large-area freestanding graphene, 50-60 GPa is the representative engineering tensile strength. The strongest material that’s ever tested is graphene. This was explained in the Nobel announcement that a cat of 4 kg weight can be supported by a graphene hammock of 1 square meter but the hammock would still be of the same weight as that of the whisker of the cat, which is 0.77 mg (about 0.001% of a 1m2 paper’s weight).
Chemical
2630 m2/g of theoretical specific surface area (SSA) is possessed by graphene. This SSA is similar to activated carbon and larger as compared to that of carbon nanotubes (CNTs) (100-1000 m2/g) and carbon black (less than 900 m2/g).
Carbon (or solid material) has many forms but graphene is the only one in which every atom can react with both of the sides chemically because of the 2-dimensional structure. Special chemical reactivity is possessed by the atoms at the graphene sheet's edges. Comparatively, the highest ratio of edge atoms is possessed by graphene than any other allotrope. Chemical reactivity is increased if there are defects within a sheet. The reaction's onset temperature between the oxygen gas and single-layer graphene's basal layer is below 530 K (260 C). The burning of graphene takes place at very fewer temperatures (620 K (350 C)). Nitrogen- and oxygen-containing functional groups commonly modify graphene; X-ray photoelectron spectroscopy and infrared spectroscopy analyze it. Although, it is a requirement of determining the graphene’s structures with nitrogen- and oxygen-functional groups that the structures should be well-controlled.
Angle based graphene
With no strain, we achieved the large-angle-bent graphene monolayer, which displayed the 2-D carbon nanostructure's mechanical robustness. Monolayer graphene can preserve its remarkable carrier mobility even with a significant amount of deformation.
Spring constant
The atomic force microscope (AFM) has been utilized for measuring the suspended graphene sheet's spring constant. The suspension of graphene sheets over SiO
2 cavities take place where an AFM tip is utilized for implementing stress to the sheet for testing the mechanical characteristics of graphene sheets. As compared to bulk graphite, the stiffness and spring constant of flat graphene sheet is different. 0.5 TPa is stiffness whereas 1-5 N/m is the range of spring constant.
Applications like resonators and pressure sensors can result because of these intrinsic characteristics. With respect to strolling, flat graphene sheets are not stable because of it’s out of plane ductility and large surface energy, for instance, bending into the shape of a cylinder, which is the lower energy state of flat graphene sheets.
Thermal conductivity
Due to their potential in applications of thermal management, a huge amount of interest has been attracted by the thermal transport in graphene, making it an active research area. Early measurements of suspended graphene’s thermal conductivity displayed remarkably huge thermal conductivity of 5300 W⋅m−1⋅K−1, in comparison with the pyrolytic graphite’s thermal conductivity of almost 2000 W⋅m−1⋅K−1 at room temperature. Although, according to the later studies on defected and scalable graphene that CVD produces, it has been seen that they can’t reproduce thermal conductivity measurements of such high levels, thus forming thermal conductivities of a broad range between 1500-2500 W⋅m−1⋅K−1 for suspended single-layer graphene.
Large ranges
Uncertainties of large measurement can cause a large range of reported thermal conductivity along with variations in the quality of the graphene and the processing conditions. Moreover, a reduction of 500 to 600 W⋅m−1⋅K−1 results in thermal conductivity at room temperature when the single-layer graphene is supported on an amorphous material due to the substrate scattering the graphene lattice waves, and for graphene of some layers covered in amorphous oxide, it can be lesser. Also, suspended graphene’s thermal conductivity can be decreased by the contribution of polymeric residue for the bilayer graphene to almost 500 to 600 W⋅m−1⋅K−1.
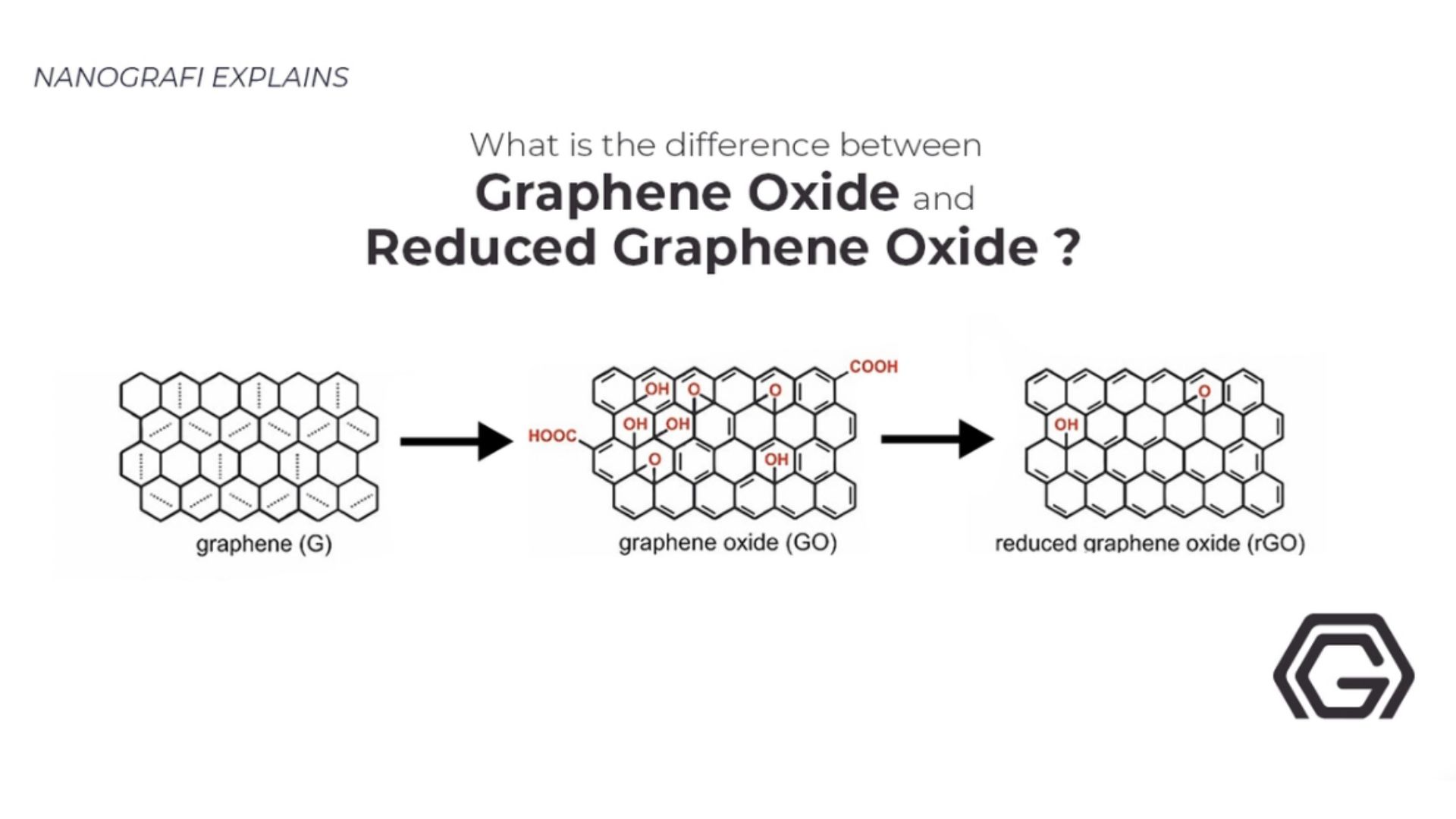
To get more information about difference between graphene oxide and reduced graphene oxide,
you can read our other blog post.
Graphene paste
In 2004, Geim et al. discovered graphene (Gr). A single layer of carbon atoms makes up graphene, with sp2 hybridization in a 2-D sheet of carbon atoms which is closely packed in a honeycomb lattice, arranged in a hexagonal configuration. When it comes to the fabrication of electrochemical sensors, Gr is an effective electrode material because of its low costs of production, strong accumulation capability, multiple possibilities of functionalization, remarkable biocompatibility, high conductivity, large specific surface, and its ability to promote the transfer of an electron between electrodes and electroactive species. In order to produce Gr-based electrochemical sensors, different strategies have been used, either by forming supramolecular architectures, by dispersion in different media, or by covalent attachment of biomolecules.
Development of electrochemical sensors
Graphene paste electrodes have been utilized for developing the electrochemical sensors for quantification of uric acid, acetazolamide, NADH, phenolic compounds, heavy metals, paracetamol, ascorbic acid, and chlorpromazine since graphene past electrode's first report in 2011. It has also been utilized as a platform to prepare a DNA sensor and an immuno-sensor for hepatitis B. In this article, the main focus is on the GrPE’s analytical application for dopamine’s highly selective quantification in the AA and serotonin’s presence by differential pulse voltammetry (DPV)-adsorptive stripping with medium exchange and to develop ethanol and phenols amperometric biosensors based on the assimilation of alcohol dehydrogenase (ADH) and its cofactor PPO (polyphenol oxidase) or NAD+, inside the composite.
Preparation of Graphene Paste Electrodes
Before the composite electrodes were made, CRGrO was grinded manually to a fine powder. We obtained the composites with ADH on mixing the corresponding mass in an agate mortar for 30 minutes for obtaining 30.0% w/w mineral oil, 10.0% NAD+, 10.0% ADH, 25.0% w/w graphite, and 25.0% w/ w CRGrO. We obtained the composite with PPO on mixing in an agate motor for 30 minutes, the mixing was 40.0% w/w mineral oil, 1.5% w/w PPO, 28.5% w/w graphite, and 30.0% w/w CRGrO. The composition was 40.0% w/w mineral oil, 30.0% w/w CRGrO, and 30.0% w/w graphite in bare GrPE’s case. On mixing of the 40.0% w/w mineral oil and 60.0% w/w graphite powder under similar conditions, classical graphite paste electrode (CPE) was achieved. Firm packing of the resulting composite was done into the Teflon tube's cavity (3mm diameter) for obtaining the corresponding electrodes. Stainless steel screw was used to establish the electrical contact. CPEs and GrPEs were polished and repacked before being used on a weighing paper.
Applications of Graphene Paste
Quantification of Dopamine
With a 2.4 mA associated current, there is a well-defined oxidation peak at 0.060 V at GrPE. However, the overvoltage for oxidation is 0.140 V more positive at CPE and the current is considerably smaller (0.014 mA), which displays the benefits of CRGrO’s presence on Do’s further electrooxidation and adsorption. A new method was developed by keeping the Do’s efficient absorption at GrPE into account for the quantification of Do based on differential pulse voltammetry (DPV)-adsorptive stripping with the medium exchange. With a saturation trend after that, there is a fast increase in the DPV-adsorptive stripping signals of up to 10.0 min. Thus for more studies in detail, the selected optimum adsorption (accumulation) time was 10.0 min.
Calibration plot
After 10 minutes of accumulation at the medium exchange and open circuit potential, DPV-adsorptive stripping provided Do's calibration plot which displayed a linear relationship between Do concentration and oxidation peak current, with 1.2 106 M quantification limit, a 4 107 M detection limit, and 43.00.4 mA Mm 1 sensitivity. It should be noted that in Do’s electrochemical quantification, a significant interferent is an AA, we evaluated the adsorptive stripping response of AA at GrPE.
Adsorptive experiments
After 10 minute accumulation in a 6.0 105 M Do solution, we performed DPV adsorptive stripping experiments at GrPE at open circuit potential, they contained AA’s increasing concentration up to 1.50 103 M, if there is no interference by AA in Do’s oxidation up to 1.0 103 M. It is mentionable that after accumulation of 10 minutes at GrPE in 1.0 103 M AA solution at open circuit, we obtain differential pulse voltammograms in phosphate buffer solution, all of which don’t display any peak, which refers to the point that the AA’s absorption is negligible under these experimental conditions. In 1.0 103 M AA's presence, the calibration plot that was obtained for Do from DPV-adsorptive stripping measurements with the medium exchange in presence of 1.0 103 M AA under the selected conditions, gave 443 mA mM1 sensitivity which is a very close value to the value that we obtained only from the Do which was 43.00.4 mA mM1, which guaranteed that even when there is a presence of large excess of AA, GrPE permits Do’s highly selective determination.
Result
One of the other exciting conclusions is that Do's selective quantification was also made possible by CRGrO’s significant electrocatalytic activity in presence of both serotonin and AA, not only in the presence of AA. 463 mA mM1 is Do's resulting sensitivity in presence of 1.0 103 M AA and 2.0 103 M serotonin, referring to the fact that even when there is a large excess presence of both compounds, Do can still be selectively quantified.
To get more information about application areas of graphene,
you can read our other blog post.
Enzymatic Biosensors Based on GrPE
Phenol Biosensing through the Usage of GrPE Modified with Polyphenol Oxidase
GrPE modified with PPO was under investigation for its analytical performance for phenolic compounds’ quantification. CRGrO’s influence on the charge transfer of quinones/catechols/phenols was evaluated for selecting the biosensor’s working conditions. Cyclic voltammograms were obtained for 1.0 103 M catechol, hydroquinone, and catechin at GrPE (solid line) and CPE (dotted line) at 0.100 V s1. In comparison with CPE, the i-E profiles that were achieved at GrPE displayed comparatively lower overvoltages and higher currents for various compounds' oxidation, thus explaining enhanced electron-transfer kinetics. Also, for the oxidation of catechin, hydroquinone, and catechol, the overvoltages decrease to 100, 70, and 300 mV.
Hydroquinone and Catechol
In hydroquinone and catechol’s case, a decrease of 88 mV and 190 mV occurs in the peak potential separation (DEp) whereas a decrease of 2.8-1.0 occurs in the cathodic-to-anodic currents ratio and for hydroquinone and catechol, a decrease of 1.3 to 1.1 occurs, which proves that CRGrO retains its remarkable electrochemical reactivity even in the presence of non-conductive mineral oils. EIS also evaluated GrPE’s electrochemical behavior. Randles circuit was used by the solid lines for representing the corresponding fitting of the data. At high frequencies, the obtained charge transfer resistances (Rct), at GrPE and CPE are 0.270.06 kW and 0.950.05 kW. For the hydroquinone/benzoquinone system, the charge transfer rate constant (k8) was attained from the EIS experiments through the usage of this equation, k ¼ RT=n2 F2 ARctC ð1Þ. In this equation, C is the redox couple concentration, A is the electroactive area, n is the number of electrons, F is the Faraday constant, T is the temperature, and R is the gas constant. At GrPE, k8 for hydroquinone/benzoquinone is 1.5 103 cm s1 and at CPE, it is 4.5 104 cm s1.
High surface reactivity
The increase in the rate constant and decrease in the Rct observed at GrPE can be the reason for high surface reactivity because of the edge defects' presence, telling the benefits of using this nanomaterial to dope CPE. The amperometric response is depicted at modified GrPE for successfully adding catechin, 5.0 106 M catechol, and corresponding calibration plots (Insets) too. After both analytes are added, we may obtain a fast and well-defined response. The analytical parameters of both catechol and catechin are discussed here. Catechin has a 2.5 107 M quantification limit, 8.2 108 M detection limit, and (1.10.1) 103 mAM1 sensitivity. Whereas, catechol has a quantification limit of 1.0 108 M, a detection limit of 3.4 109 M, and a sensitivity of (1.380.02) 104 mAM1. In the tea bag, GrPE-PPO was utilized for catechin’s quantification without any pretreatment, being 1.90.4 % w/v per bag.
Ethanol Biosensing Using GrPE Modified with NAD+ and ADH
An ethanol enzymatic biosensor was made by using GrPE as a platform and through NAD+ and ADH’s incorporation within the composite. The overvoltage faces a major decrease at GrPE for NADH's oxidation and also there is a significant improvement in the oxidation currents, all of which explains the fact that the electrooxidation of NADH is largely improved by CRGrO’s presence. For further work, the selected potential was 0.450 V. Amperometric recordings are displayed for successful additions of ethanol of 1.0 103 M. In comparison with the previously shown hydrodynamic voltammogram, the oxidation current is negligible at CPE-ADH-NAD+. An extremely sensitive response is obtained at GrPE-ADH-NAD+ at variance with this behavior.
Quantification of Ethanol
As compared to the other similar biosensing strategies, this biosensor enables ethanol’s successful quantification in an extremely easy, sensitive, and fast way with enhanced detection limits. Here, a remarkable analytical performance was obtained with an extremely simple scheme by mixing ADH/NAD+ with GrPE, even when there were other methods to be used as the association of cross-linking agents, ionic liquids, polymers, CNTs, and Gr that displays lower detection limits. Various alcoholic beverages were utilized to challenge the biosensor. According to the results, ethanol’s quantification in Benjamin Nieto Senetiner white wine, Bacardi gold rum, and Mumm champagne is made possible by the biosensor with no treatment and with a very good correlation with the values.
Results
According to the results, it is the GrPE that combines the composite materials’ versatility with the benefits of the CRGrO’s effective electrocatalytic activity, offering a beneficial avenue for bio-sensing and electrochemical sensing of various analytes. Gr’s catalytic activity and the Do’s efficient and preferential adsorption at GrPE made Do quantification possible at submicromolar levels even in the presence of high excess of serotonin and ascorbic acid.
Incorporative methods
PPO and ADH/NAD+ incorporation inside the GrPE displays a simple and fast way for designing new enzymatic biosensors for the quantification of ethanol and phenols, based on Gr’s catalytic activity towards NADH’s oxidation and quinones’ reduction. CRGrO’s incorporation inside the CPE gives a great alternative in novel analytical platforms’ development to detect various bio-analytes in a fast yet simple way.
Conclusion
Graphene powderis highly used for dopamine, phenol, and ethanol. Dopamine is used for the functioning of various systems of the human body while ethanol is used for clinical and forensic purposes. However, phenol is used for the agricultural and manufacturing industries. All these three compounds play a huge role in making human life better so graphene powder in return plays a huge role in making these compounds better for usage.
To get more information, you can visit our Blografi.
References:
https://cutt.ly/ZHoGmAc
https://www.sciencedirect.com/science/article/pii/S1388248111000403
https://academic.oup.com/nsr/article/5/1/90/3861355
https://cutt.ly/jS0YgOn
Recent Posts
-
Advanced Materials for Unmanned Aerial Vehicle (UAV) Protection Against Laser
Consider a UAV on a critical mission, rendered inoperative by a sudden laser attack. With the increa …26th Jul 2024 -
Simulation and Modeling of Material Properties
Our world is composed of a dazzling array of materials, each with its own unique properties that dic …19th Jul 2024 -
Advanced Coatings for Superior Corrosion and Wear Resistance
Corrosion and wear pose significant challenges across various industries, leading to substantial eco …12th Jul 2024