Perovskites: Revolutionizing Energy Conversion Technologies - Nanografi
Perovskite structured materials, also known as perovskites, are a class of compounds that have a crystal structure similar to the mineral perovskite. They exhibit excellent charge transport properties, high absorption coefficients, and long carrier diffusion lengths.
They exhibit excellent charge transport properties, high absorption coefficients, and long carrier diffusion lengths. These materials have gained significant attention in various fields, including photovoltaics, light-emitting diodes (LEDs), sensors, and catalysts. Perovskites have shown promising potential for next-generation solar cell technology due to their ability to efficiently convert sunlight into electricity. At Nanografi, we offer you these promising materials for future technologies and continue to work every day to contribute to the scientific community.
Introduction
Perovskite materials are composed of an inorganic framework and can contain a variety of elements. The general chemical formula for perovskite structured materials is ABX3, here A and B are positively charged ions called cations and X is negatively charged ion called anion. The A-site is typically a larger cation, while the B-site is usually a smaller cation. The X-site can be an anion such as oxygen, halides (chloride, bromide, iodide), or a combination of elements. Perovskite structured materials have gained significant attention in recent years, particularly in the field of photovoltaics, due to their remarkable optoelectronic properties. Specifically, metal halide perovskites, such as methylammonium lead iodide (CH3NH3PbI3), have demonstrated high power conversion efficiencies in solar cells. These materials have shown the ability to efficiently convert sunlight into electricity, making them promising candidates for next-generation solar cell technology.
Perovskite materials also find applications in other areas such as light-emitting diodes (LEDs), photodetectors, lasers, sensors, and catalysts. They exhibit excellent charge transport properties, high absorption coefficients, and long carrier diffusion lengths, which make them attractive for various optoelectronic and energy-related applications. However, it is important to note that perovskite materials face challenges related to stability and durability. They are sensitive to moisture, heat, and light, which can lead to degradation over time. Researchers are actively working to improve the stability and reliability of perovskite materials for commercialization. Overall, perovskite structured materials hold great promise for a wide range of applications, particularly in the field of renewable energy, and ongoing research and development efforts aim to overcome the existing limitations and unlock their full potential.
Perovskite refers to a class of materials that have a specific crystal structure, named after the mineral perovskite (calcium titanium oxide). Perovskite materials have a unique arrangement of atoms in a cubic lattice, with a general chemical formula of ABX₃, where A and B represent different cations, and X represents an anion. Perovskite materials gained significant attention in the scientific community, particularly in the field of solar energy, due to their remarkable properties. In recent years, perovskite solar cells have emerged as a promising alternative to traditional silicon solar cells. Here are a few reasons why perovskite is important:
- High efficiency: Perovskite solar cells have shown the potential to achieve high power conversion efficiencies comparable to or even surpassing traditional silicon-based solar cells. They can convert a significant portion of sunlight into electricity.
- Low-cost fabrication: Perovskite solar cells can be produced using low-cost and scalable manufacturing techniques such as solution processing, which makes them potentially more cost-effective than silicon-based solar cells. This could lead to reduced manufacturing and installation costs for solar energy systems.
- Versatility: Perovskite materials can be easily synthesized with different compositions by varying the elements A, B, and X in their chemical formula. This versatility allows for tailoring their optical and electronic properties, making them suitable for various applications beyond solar cells. Perovskites have been explored for use in light-emitting diodes (LEDs), photodetectors, sensors, and other optoelectronic devices.
- Tunable characteristics: Perovskite materials have outstanding optoelectronic characteristics, such as strong light absorption, lengthy charge carrier diffusion lengths, and effective charge transport. Through material engineering and device architectural design, these attributes may be further enhanced and optimized, providing chances to increase device performance.
What is Perovskite Structure?
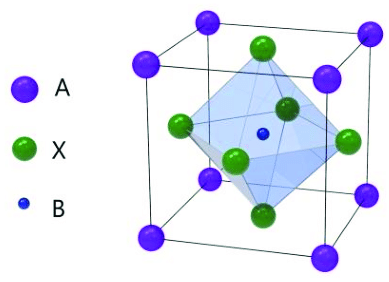
In the perovskite structure the "A" cation is typically larger and located at the corners of the crystal structure, while the smaller "B" cation is situated at the center of each face of the structure. The "X" anion is positioned at the center of the structure, surrounded by the cations.
In perovskite materials, the arrangement of atoms creates a cubic unit cell, which is the basic repeating unit of the crystal structure. This unit cell consists of a framework of corner-sharing octahedra, formed by the "X" anions and the surrounding cations. The "A" cations occupy the voids between these octahedra, and the "B" cations occupy the octahedral sites. One of the most well-known perovskite materials is perovskite solar cells, which have gained significant attention for their potential use in photovoltaic applications. These solar cells typically use organic-inorganic hybrid perovskite materials, such as methylammonium lead iodide (CH3NH3PbI3), which exhibit excellent light-absorbing properties.It's crucial to keep in mind that perovskite structures can also show deviations and distortions from the ideal cubic structure, resulting in various crystal symmetries. The characteristics and behavior of the material may be significantly impacted by these aberrations. Perovskite materials have found use in a variety of industries, including superconductivity, catalysis, solar cells, fuel cells, and more. Perovskite structures are a topic of ongoing research because they have a special mix of qualities and the capacity to modify them.
Types and Structures of Perovskite Materials
Several researchers have tried to categorize the perovskite-type formations according to the radii of the component metallic ions. due to the ABO3 perovskite's adaptability to a variety of cations with various oxidation states, as well as its capacity to do so.
The main property of perovskites is the possibility of multiple cation substitutions, which results in the occurrence of large groups of compounds with different cations in the B position (ABxB1xO3), different cations in the A position (AxA1xBO3), and with substitution in both cation positions (AxA1xBxB1xO3).According to Modeshia and Walton (2010), the perovskite complex type A (Bx′By′′) O3 compounds may be split into the following four subgroups:
- (Bx′By′′) O3z compounds, which have oxygen-deficient phases.
- Alternatives that only include A (B′0.5B′′0.5) O3 and equal proportions of the two B components.
- Those that have A (B′0.33B′′0.67 O3, a higher valence state element, are twice as valuable as A (B′0.67B′′0.33) O3, a lower valence state element.
- Those that contain A (B′0.67B′′0.33) O3, a higher valence state element, are twice as valuable.
Perovskite materials exhibit a number of fascinating properties as a result of their unique chemical composition, like non-stoichiometry of the anions and/or cations, a combined valence electronic framework, distortion of the cation arrangement, and mixed valence. By partially replacing cations in places A and B in perovskites, a variety of complex types with unusual properties, such as dielectric characteristics, transparency, ferroelectricity, superconductive properties, piezoelectricity, multiferroicity, and catalytic activity, can be created.
ZnTiO3: Structure and Properties
Since 1960, several researchers have published fundamental research applicable to the phase diagram and the characterization of the ZnO-TiO2 combination. They claimed that the ZnO-TiO2 system contains three different compounds: Zn2TiO4 which is cubic, ZnTiO3 which is hexagonal, and Zn2Ti3O8 which is also cubic. ZnTiO3 has an oxide structure like perovskite and was a potential contender for application as a paint pigment, gas sensor (for ethanol, NO, and CO, among other things), and microwave resonator. An alternative of ZnTiO3 is Zn2Ti3O8 which has very low temperature. However, it has not been possible to create unadulterated ZnTiO3 from a combination since the compound decomposes at a temperature of around 945 °C into rutile and -Zn2TiO4.ZnTiO3 powder may be made in a variety of techniques, including solid state reactions. The sol-gel approach is used to create zinc titanate nano-crystalline powders; however, the procedures are often difficult and costly chemicals are required. The typical solid-state process, which is easier to run and employs affordable and readily accessible oxides as starting ingredients, has been tried to create ZnTiO3 powders. Additionally, the properties of the formed ZnTiO3 powders and the reaction's kinetic behavior were looked at.
BaTiO3: Structure and Properties
Barium titanate has the chemical formula BaTiO3. It has a perovskite structure and ranges in color from white to grey when in powder form. It is soluble in a variety of acids. It is insoluble in both alkalis and water. The material will exhibit an increase in resistivity at a particular temperature, referred to as the Curie temperature, with the increase frequently being many orders of magnitude. The Curie temperature can be controlled to some extent by the dopant. At the Curie temperature, barium titanate transitions from a tetrahedral to a cubic phase. Additionally, it has been found that the properties of NTCR (negative temperature co-efficient of resistivity) in barium titanate single crystals.
SrTiO3: Structure and Properties
The mineral that bears the same name, calcium titanate (CaTiO3), also has a perovskite structure. Strontium titanate (SrTiO3) does. It has a cubic shape at ambient temperature, but as it becomes colder than 105K, it takes on a tetragonal shape. Strontium titanate has superconducting and piezoelectric properties at very low temperatures. Additionally, strontium titanate has a very high dielectric constant.
Perovskite Nanowires and 2D Materials:
In the past few years, especially in the field of energy, there has been a great deal of interest in the extraordinary optoelectronic properties of halide perovskites. When the dimension of halide perovskites lowers, new properties such as a bigger band gap, stronger photoluminescence quantum yield, and larger exciton binding energies will become apparent. Low-dimensional halide perovskites, in particular two-dimensional (2D) halide perovskites that have attracted a lot of attention lately because of their potential for usage in photodetectors, light-emitting diode (LEDs), and solar cells. Following this, several methods for creating 2D halide perovskites were developed, and their intriguing optoelectronic properties were examined. The optoelectronic devices built on 2D halide perovskites have demonstrated excellent performance, including highly sensitive photodetection, robust electroluminescence with unique emission peaks, and ecologically stable solar cells with acceptable power conversion efficiency. The industrial standard for perovskites used in research in this case is 2D halide perovskites.
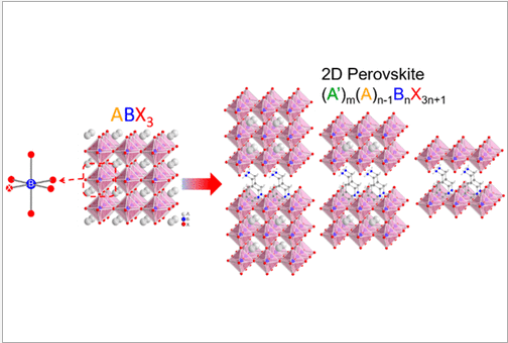
Perovskite-Type Super Lattices:
Low-dimensional metallic perovskites made of halide with sequential inorganic-organic architectures have stood out for their remarkable stability with hysteresis-free electrical properties in comparison to their 3D (three-dimensional) counterparts. The distinctive multiple-quantum-well structure of the polycrystals, with its randomly aligned quantum wells and grain boundaries, limits the device's efficiency. In single crystals, stacked organic spacers that act as insulators stop carrier transmission in the thickness direction. The strong quantum confinement brought on by the biological spacers also limits the production and movement of free carriers. Lead-free metal halide perovskites are also produced; however, they exhibit poor device performance due to their unstable structure and low crystallinity. Here, we report a superlattice of the low-dimensional metal halide perovskite BA2MAn1SnnI3n+1 (BA, butylammonium; MA, methylammonium) created using chemical epitaxy. The vertical alignment of the inorganic slabs with the substrate and their interconnection in a crisscross 2D network that runs parallel to the substrate enables efficient carrier movement in three dimensions. The substrate's mismatched lattice compresses the organic spacers, reducing the amount of quantum confinement. The quasi-steady state performance of a superlattice solar cell has been confirmed, showing a stable 12.36% photoelectric conversion efficiency. Additionally, an intraband exciton relaxation process may have generated a very high open-circuit voltage (VOC).
Applications of Perovskite Materials
Perovskite Materials for Energy and Environmental Applications
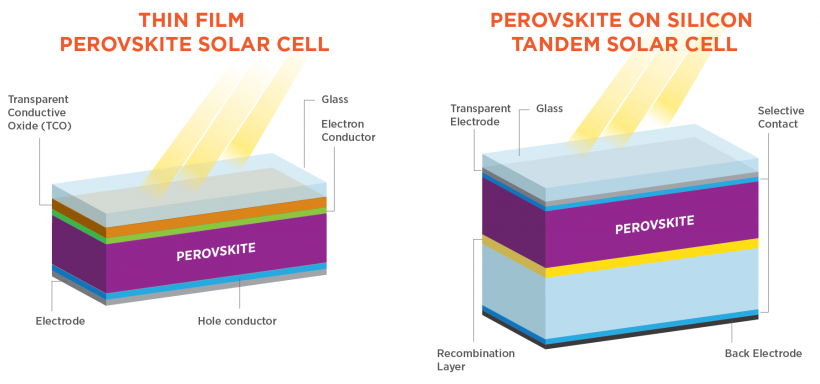
A perovskite solar cell (PSC) is a type of solar cell that employs a compound with a perovskite structure as the light-harvesting active layer. These materials are often based on lead or tin halides and are hybrid organic/inorganic materials. Methylammonium lead halides and completely inorganic cesium lead halides are two examples of affordable and simple to produce perovskite materials. In tandem cells built on silicon, the efficiency climbed to 29.8%, surpassing the greatest efficiency attained in single-junction silicon solar cells, while in single-junction designs, it grew from 3.8% in 2009 to 25.7% in 2021. Perovskite solar cells, therefore, reflected the solar technologies that were evolving the quickest as of 2016. Perovskite solar cells are growing increasingly popular on the market as a result of their incredibly low production costs and potential for even higher efficiency. Key concerns and research subjects related to both their immediate and long-term stability.
Increasing the Stability and Durability of Perovskite Solar Cells
For perovskite solar cells to be used in real applications, the issue of long-term stability must be solved. Materials science can contribute to advancements in stability and scalability toward allowing large-scale manufacturing through developing synthetic chemistry, materials characterization, and device engineering. Three interrelated themes—characterizing instability, creating stable perovskites, and healing the interfaces are mostly discussed.
Tin-Based Perovskite Solar Cells
Tin-based perovskite solar cells are a special type of perovskite solar cell in which tin is used in place of lead. It has a tin-based perovskite structure. Methylammonium tin triiodide has a band gap of 1.2–1.3 eV compared to formamidinium tin triiodide. Tin-based perovskite solar cells are still in the research phase and have received very few papers in contrast to their lead-based version.The unstable nature of the tin's 2+ oxidation state in methylammonium tin iodide, which is quickly changed to the more stable form and causes self-doping, is the main cause of this, which affects solar cell efficiency. Self-doping was traditionally assumed to be the result of inherent vacancy faults, but recent research reveals this assumption may not be totally accurate. Cs vacancies are the primary reason for the holes that lead to self-doping in CsSnI3.
Lead-free Bismuth-Based Materials for Solar Cell
Materials based on bismuth can make intriguing replacements for compounds that include lead. The price of bismuth is relatively low and consistent due to its abundance in the earth's crust, the fact that it is a by-product of the refining of Pb, Cu, and Sn, and the fact that it has few substantial economic applications. Bismuth is also regarded to be non-toxic although being a heavy metal, and it is even an ingredient in well-known drugs like Pepto-Bismol. Additionally, Bi3+ has been hailed as a leading candidate for defect-tolerant compounds, or materials with potent optoelectronic properties even in the presence of faults. The active ns2 lone pair likes to create antibonding interactions close to the valence band maximum, which, in principle, limits defects to shallow states at the band edges.
Perovskite Materials for Biomedical Applications
In recent years, solution-processable hybrid perovskites have achieved extraordinary performance on solar cells and photodetectors thanks to their exceptional light absorption and improved carrier transport capabilities. Hybrid perovskites are ideal for X-ray imaging and detection because they include a high atomic number of lead and halide elements. Low-dose pictures and perovskite-based X-ray detectors are extremely useful in medical settings. Furthermore, double-perovskite for bovine serum albumin adsorption and magnetic oxide perovskite nanoparticles with magneto-temperature response is significant. Additionally, investigations on in vitro cell proliferation show that perovskite CaTiO3 has a potential biocompatibility in cellular settings and low cytotoxicity, which support its beneficial activities in osseointegration and osteoblast adhesion.
Perovskite Materials for Optoelectronic Applications
Solution-processable hybrid perovskites have recently achieved amazing performance on solar cells and photodetectors thanks to their enhanced carrier transport capabilities and extraordinary light absorption. Hybrid perovskites are excellent candidates for X-ray detection and imaging due to their high atomic number lead and halide content. Low-dose pictures and perovskite-based X-ray detectors have several medicinal uses. Also crucial is the double-perovskite for the adsorption of bovine serum albumin and the magnetic oxide perovskite nanoparticles with a magneto-temperature response. Furthermore, investigations on in vitro cell proliferation show perovskite CaTiO3 to have low cytotoxicity and a promising biocompatibility in cellular settings, supporting its beneficial effects in osseointegration and osteoblast adhesion.
Light-Emitting Diodes and Lasers:
Halide perovskites-based light-emitting diodes have developed quickly in the last decade and can currently offer outer quantum efficacy of over 23%. The low efficacy of blue-emitting devices, the difficulty of accessing emission wavelengths above 800 nm, a reduction in outside quantum effectiveness at a high current density, a partial understanding of the impact of an electric field on mobile ions evident in the perovskite materials, and short device lifetimes limit the practical use of such devices. The development of reliable and effective gadgets is not without its difficulties. Utilize emissions in the long infrared range and create spin-polarized light-emitting diodes to overcome the limited efficacy of blue-emitting devices.
Future of Perovskite Materials
Because of its stable structure, an abundance of compounds, and variety of characteristics, perovskite oxides offer a wide range of uses. Due to its many compounds, extremely stable structure, range of characteristics, and several useful uses, inorganic perovskite type oxides are an appealing nanomaterial for a variety of applications. Some of these substances are widely used as nanomaterials in the catalysis of numerous chemical generating sectors. These oxides perform better as catalysts than any other oxides of transition and precious metals. Due to the distinctive assortment of characteristics of perovskite oxides, thin film capacitors, non-volatile storage, photo-electrochemical cells, and other applications have become feasible. enduring memories, transducers, actuators, and sensors, drug delivery, modern chemical industry catalysts, ultrasonic waves & undersea devices, and ultrasonic imaging. documenting software, Hard disk read heads, spintronics devices, laser beam applications, windows that prevent infrared radiation at elevated temperatures, and high-temperature heating applications. finishes with heat barriers.
Conclusion
Due to their remarkable qualities and possible uses, perovskite materials have attracted a lot of interest in the fields of optoelectronics and renewable energy. A family of minerals known as perovskites has a particular crystal structure that is frequently described as ABX3, where A and B are cations and X is an anion. Methylammonium lead iodide (CH3NH3PbI3) is the perovskite substance that is most often researched.
The exceptional photovoltaic qualities of perovskite materials are one of its main promises. Perovskite solar cells now rival or even outperform conventional silicon-based solar cells in terms of their impressive power conversion efficiencies (PCEs). Their superior light-absorbing abilities, lengthy carrier diffusion lengths, and strong charge carrier mobilities are the main causes of this. Perovskite solar cells may also be produced utilizing low-cost solution-based techniques, possibly increasing their affordability and scalability. Perovskite materials are appealing for light-emitting applications such as light-emitting diodes (LEDs) and display technologies since they also provide efficient luminescence. Perovskite LEDs may be made with a variety of colors thanks to their adjustable bandgap and robust emission, which may result in high-quality and energy-efficient displays.
In summary, perovskite materials hold great promise for a wide range of applications in the field of optoelectronics and renewable energy. Continued research and development efforts are focused on improving the stability, scalability, and sustainability of perovskite devices, with the ultimate goal of realizing their commercial potential and contributing to a more sustainable and efficient future.
Nanografi engages in comprehensive research and development in the renewable energy sector, delivering groundbreaking solutions to boost sustainability.
References
Barium Titanate (BaTiO3) - Properties and Applications. (n.d.). Retrieved March 20, 2024, from https://www.azom.com/article.aspx?ArticleID=2280
Bati, A. S. R., Zhong, Y. L., Burn, P. L., Nazeeruddin, M. K., Shaw, P. E., & Batmunkh, M. (2023). Next-generation applications for integrated perovskite solar cells. Communications Materials 2023 4:1, 4(1), 1–24. https://doi.org/10.1038/s43246-022-00325-4
Fakharuddin, A., Gangishetty, M. K., Abdi-Jalebi, M., Chin, S. H., bin Mohd Yusoff, A. R., Congreve, D. N., Tress, W., Deschler, F., Vasilopoulou, M., & Bolink, H. J. (2022). Perovskite light-emitting diodes. Nature Electronics 2022 5:4, 5(4), 203–216. https://doi.org/10.1038/s41928-022-00745-7
Gong, J., & Xu, T. (2020). Perovskite Materials in Biomedical Applications. Materials Horizons: From Nature to Nanomaterials, 95–116. https://doi.org/10.1007/978-981-15-1267-4_4
Lei, Y., Li, Y., Lu, C., Yan, Q., Wu, Y., Babbe, F., Gong, H., Zhang, S., Zhou, J., Wang, R., Zhang, R., Chen, Y., Tsai, H., Gu, Y., Hu, H., Lo, Y. H., Nie, W., Lee, T., Luo, J., … Xu, S. (2022). Perovskite superlattices with efficient carrier dynamics. Nature 2022 608:7922, 608(7922), 317–323. https://doi.org/10.1038/s41586-022-04961-1
Mao, L., Stoumpos, C. C., & Kanatzidis, M. G. (2019). Two-Dimensional Hybrid Halide Perovskites: Principles and Promises. Journal of the American Chemical Society, 141(3), 1171–1190. https://doi.org/10.1021/JACS.8B10851/ASSET/IMAGES/MEDIUM/JA-2018-10851V_0014.GIF
Perovskite - Wikipedia. (n.d.). Retrieved March 20, 2024, from https://en.wikipedia.org/wiki/Perovskite#Perovskit...
Perovskite Solar Cells | Department of Energy. (n.d.). Retrieved March 20, 2024, from https://www.energy.gov/eere/solar/perovskite-solar-cells
Photonic crystals for perovskite‐based optoelectronic applications - Yang - 2022 - Nano Select - Wiley Online Library. (n.d.). Retrieved March 20, 2024, from https://onlinelibrary.wiley.com/doi/10.1002/nano.202100163
Strontium Titanate (SrTiO3) - Properties and Applications. (n.d.). Retrieved March 20, 2024, from https://www.azom.com/article.aspx?ArticleID=2362
Tao, S., Polavarapu, L., & Vivo, P. (2023). Perovskite photovoltaics: stability and scalability. Scientific Reports 2023 13:1, 13(1), 1–3. https://doi.org/10.1038/s41598-023-31512-z
The structure of perovskite with ABX3 general formula. Eight [BX6]... | Download Scientific Diagram. (n.d.). Retrieved March 20, 2024, from https://www.researchgate.net/figure/The-structure-of-perovskite-with-ABX3-general-formula-Eight-BX6-octahedras-surround_fig5_343684797
Yang, X., Yang, H., Hu, X., Li, F., & Yang, Z. (2022). Photonic crystals for perovskite-based optoelectronic applications. Nano Select, 3(1), 39–50. https://doi.org/10.1002/NANO.202100163
Recent Posts
-
Reducing the Carbon Footprint of Nanomaterials
The production of nanomaterials is vital for numerous advanced applications, from healthcare to elec …26th Apr 2024 -
Nanocomposites in Food Packaging
The utilization of nanocomposites in food packaging represents a significant advancement in the fiel …19th Apr 2024 -
What is the Difference Between 7075 and 6061 Aluminum Alloy?
When comparing 7075 aluminum alloy to 6061 aluminum alloy, it's essential to understand their disti …5th Apr 2024






