Comprehensive Guide to Water Soluble Quantum Dots
Small semiconductor nanoparticles known as water-soluble quantum dots have the capacity to emit a variety of colored light when excited. Because they can be disseminated in aqueous solutions, they are referred to as "water-soluble," and this makes them easier to work with in some applications than conventional, insoluble quantum dots.
They are employed in a number of industries, including medical, imaging, and displays. They are designed to dissolve in water, making them simpler to work with in watery conditions. They are valuable in a range of applications, including as bioimaging, sensing, and solar cell technology, because to their distinctive optical features, such as high fluorescence. Elevate your research with Nanografi's water soluble quantum dot products, quintessential for a myriad of analytical applications.
Introduction
For biolabeling applications and other purposes that call for stable dispersion of these fluorescent nanocrystals in aqueous solutions, water-soluble Quantum Dots (QDs) are particularly helpful. The hydrophilic monolayer-coated hydrophilic surfactant coats the outer surfaces of compact, water-soluble QDs. With the help of these surfactant coatings, the QDs can develop a hydration shell that inhibits aggregation and encourages dispersion.
What are Water Soluable Quantum Dots?
Water Soluble Quantum Dots are defined by their ability to disperse in water-based solutions, offering greater convenience over traditional, non-soluble counterparts. Compact water-soluble quantum dots are available from Nano optical Materials coated with a selection of three hydrophilic surfactants, each of which has a unique terminal function group that enables the use of various conjugation chemistries to attach the QDs to biomolecules in order to produce fluorescent probes and label surfaces.Among the options for terminal functional groups are diols, primary amines, and carboxylic acids (hydroxyl groups on adjacent carbon atoms).
All of the organic soluble Quantum Dots provided, including the Cd-free, non-toxic CuInZnS/ZnS QDs, are available in The low stability of quantum dots in buffers and physiological liquids is a recurring problem that has restricted the use of these particles in biology and medicine. This restriction is not present in water-soluble quantum dots, making them ideal for biomedical research. These particles don't photobleach and are stable under physiological settings. Since most proprietary technology permits the incorporation of PEG spacers (which restrict the non-specific binding to these particles) and a number of functional groups on the surface of these particles, they are the ideal choice for bio-labeling and bio-conjugation. To assure their quality, each batch of water-soluble quantum dots is characterized using TEM, UV-Vis, and fluorescence water-soluble forms.
Figure 1. Multicolored Water-soluble Quantum Dots.
Water Soluble Graphene Quantum Dots
A type of zero-dimensional flat graphenic material with lateral diameters lower than 10 nm is known as graphene quantum dots (GQDs). It has been demonstrated that water-soluble GQDs offer potential in a number of biological applications, including bioimaging, sensing, and biological treatment. The large heterogeneity in these water-soluble GQDs' compositions, sizes, and shapes, however, poses significant difficulties for better understanding the relationship between their structure and properties as well as for expanding clinical applications in terms of large-scale manufacturing procedures and security issues.
On the other hand, by employing a logical organic pathway and the dehydrocyclization of dendritic polyphenylenes, GQDs with precise and unambiguous structures can be produced. Since the majority of these well-defined organically synthesized GQDs are naturally water-insoluble and prone to aggregate into stacked large particles due to strong interactions between the nano-scale planar carbon skeleton, they are rarely used in biology. Nevertheless, they have demonstrated applications in electronics, optics, and energy conversion.
Soluble Graphene Quantum Dot for Cancer Sonodynamic Therapy
The development of a generic synthetic method to render these atomically accurate GQDs soluble and monodispersed in water is urgently needed in order to produce a variety of well-defined nanomaterials for bio-applications, such as sonodynamic treatment (SDT). SDT is a highly effective and non-invasive anti-cancer treatment. The sonochemical process generates cytotoxic reactive oxygen species (ROS) to harm the tumor during SDT, as opposed to hydrodynamic stress and cavitation. More crucially, the addition of US-responsive sensitizers (sonosensitizers) to the sonochemical process could significantly improve SDT efficacy."
Traditional molecular sonosensitizers produce ROS at relatively low rates and experience chemical instability at US circumstances due to self-destruction caused by ROS. Sonosensitizers made of nanomaterials show good stability and increased ROS production. Despite the apparent advantages of nanomaterial sonosensitizers, most lack clearly defined structures. For the advancement of SDT, novel sonosensitizers with well-defined small molecule structures and superior sonosensitization efficacies of nanomaterials are essential.
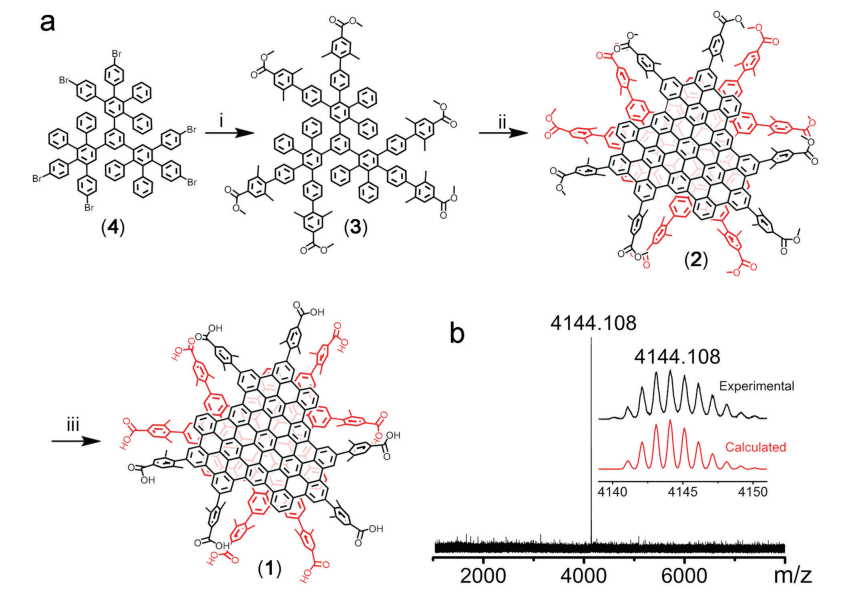
Figure 2. Synthesis of WAGQD-C96.
The study investigated how sterically hindered water-soluble functional groups were used to create an atomically accurate, water-soluble GQD, known as WAGQD-C96. how sterically hindered water-soluble functional groups were used to create an atomically accurate, water-soluble GQD, known as WAGQD-C96. Mass, NMR, and UV-vis spectroscopies were used to analyze WAGQD-C96's clearly defined chemical and assembly structures. The monodispersion of WAGQD-C96 in water was confirmed by high-resolution transmission electron microscopy (HRTEM) and dynamic light scattering (DLS) without additional aggregation. The deep-red emission of WAGQD-C96 was used to investigate the biological characteristics of the compound, including cellular uptake, cellular SDT effects, and retention in tumor. The ability of WAGQD-C96 to produce more ROS under US irradiation than normal organic molecular sonosensitizers has been demonstrated. According to in vivo research, WAGQD-C96 can significantly reduce tumor growth in terms of SDT without clearly manifesting any harm.
Water Soluable Silicon Quantum Dots (SiQDs)
According to research, carboxy-terminated silicon quantum dots (SiQDs) are highly water soluble due to their high molecular coverage of surface monolayers, bright light emission with high photoluminescence quantum yields (PLQYs), long-term stability in the PL property for monitoring cells, low toxicity to the cells, and a high photothermal response. Through the thermal disproportionation of triethoxysilane hydrolyzed at pH 3 and subsequent hydrofluoric etching, created water-soluble SiQDs by thermally hydrosilylating 10-undecenoic acid on their hydrogen-terminated surfaces.
Biocompatibility and Therapeutic Potential of SiQDs
In hydrophilic solvents like ethanol and water, the SiQDs functionalized with 10-undecanoic acid (UA:SiQDs) displayed long-term stability (pH 7). Cellular absorption, short-term toxicity, and, for the first time, long-term cytotoxicity were used to evaluate their impact on live cells. With their excellent optical properties that allow imaging for more than 18 days and a photothermal response with a 25.1% photothermal conversion efficiency in addition to the clear evidence of cell death by laser irradiation, the results demonstrate that UA:SiQDs are potential candidates for theranostics. With full viability of up to 400 g/mL for a short period of time and a 50% cell viability value after 14 days of incubation at a concentration of 50 g/mL, UA:SiQDs exhibit negligible cytotoxicity.
Water Soluble Quantum Dots for Multiphoton Fluorescence Imaging
Multiphoton microscopy's use of semiconductor nanocrystals (quantum dots) as fluorescent labels allows for multicolor imaging in challenging biological contexts like live tissue. For multiphoton imaging in living animals, they characterized water-soluble cadmium selenide-zinc sulphide quantum dots. These fluorescent probes have the greatest two-photon action cross sections of any label used in multiphoton imaging, reaching up to 47,000 Goeppert-Mayer units. They dynamically viewed quantum dots in capillaries hundreds of micrometers deep through the skin of living mice. Bright, photostable fluorophores known as quantum dots (QDs) have a limited Gaussian emission spectrum but a wide excitation range. Wavelengths that are subject to material size restriction. QDs make it possible to image biological samples in multiple colours effectively. They should be especially helpful for fluorescence imaging in living tissues, where signals might be masked by conflicting intrinsic emissions and scattering. Multiphoton microscopy has replaced wide-field or confocal microscopy as the principal fluorescence imaging method in thick specimens because it allows for deep imaging of a range of biological samples with less overall photobleaching. Researchers have looked into the two-photon excitation properties of QDs and have started to research their potential for in vivo multiphoton imaging in order to tackle these challenging imaging challenges.
Biological applications require strong, water-soluble QDs. Several synthetic techniques have been employed, including block-copolymer micelles, salinization, and surface functionalization using water-soluble ligands. Here, they looked at the photophysical characteristics of CdSe-ZnS nanocrystals made by encapsulating them in an amphiphilic polymer, which are water-soluble. A variety of organic and water-soluble QDs in various colors had their two-photon action cross sections determined. The action cross sections, which offer a direct measure of brightness for imaging, are created by multiplying the fluorescence quantum efficiency (f) by the nonlinear two-photon absorption cross section 2P. Depending on the specific preparation and excitation wavelength, the recorded values varied from 2000 to 47,000 Goeppert-Mayer units (GM).
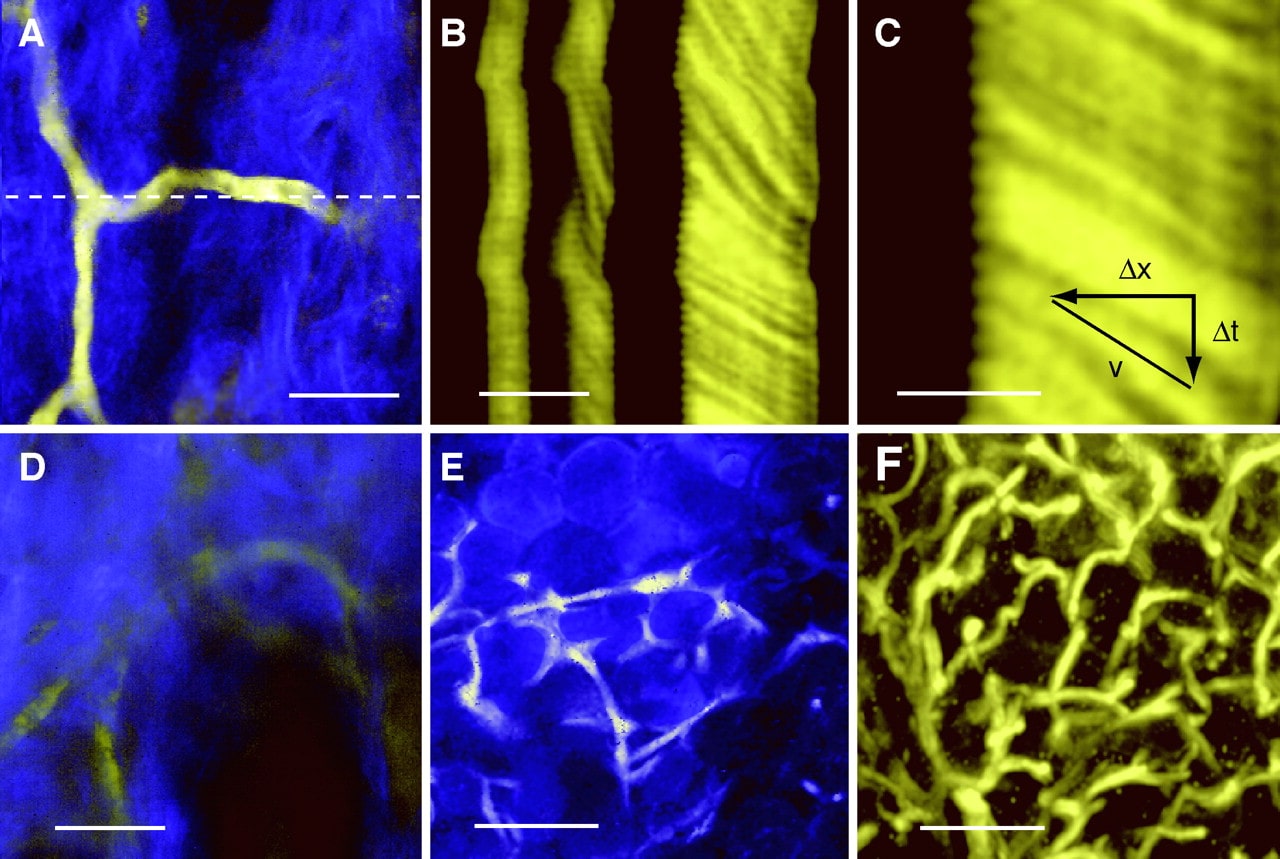
Figure 3. Water-Soluble Quantum Dots for Multiphoton Fluorescence.
The biological behaviors of WAGQD-C96 can be studied because it is water soluble, and its deep-red emission makes it simple to do so. Confocal laser scanning microscopy (CLSM) was used in cellular level experiments and monitored, demonstrating that WAGQD-C96 displayed time-dependent cellular uptake. Analyses using flow cytometry revealed that WAGQD-C96 was taken up similarly by cells Supporting Information). The WAGQD-deep-red C96's emission can also be employed for tumor visualization and fluorescence diagnostics. The FL pictures and estimated mean intensities showed a satisfactory photoemission and concentration-dependent connection of WAGQD-C96.Following intratumoral injection, the time-dependent retention of WAGQD-C96 in the tumor area was visually examined. It was shown that WAGQD-C96 was retained in tumor tissue for a very long duration, with most signals remaining for up to 72 hours. All animal tests were conducted in accordance with the Xiamen University Laboratory Animal Center's approved protocols (XMU2021101).
Quantum dots (QDs), fluorescent semiconductor nanocrystals, exhibit various distinctive optical and chemical properties. These qualities make them appealing fluorescent tags for biological applications involving cell and developmental processes that need for long-term, multi-target, and extremely sensitive imaging. The current surge in the use of QDs in biological imaging is a result of improved water-stable QD production, methods for labelling cells effectively with QDs, and advancements in conjugating QDs to certain proteins. Although there have been many triumphs employing QDs for biological applications, there are still obstacles that need to be removed before biologists may regularly use these potent tools.
For more information about quantum dots, read our blog post.
Uses of Water Soluable Quantum Dots
Water-soluble quantum dots (QDs) are nanocrystals with diverse applications, particularly in biomedical fields due to their unique optical properties. They are used as sensitive fluorescent probes in bioanalytical applications, leveraging their photostability for long-term imaging in biological systems. Their biocompatibility and optical stability are achieved through environmentally friendly synthesis methods. In microscopy, they enable multicolor imaging in live tissues with minimal photodamage. Water-soluble QDs also contribute to chemical analysis, such as measuring urea in blood serum, and serve as photocatalysts in chemical reactions. Beyond biomedical uses, QDs are incorporated into electronic devices like flat-screen displays and are explored for nano- and microscale engineering, showcasing their wide-ranging utility across multiple industries.
Biomedical Applications of Water Soluble Quantum Dots
With unique size-dependent optical and electrical features brought on by quantum confinement, semiconductor nanocrystals are inorganic particles that are 1–10 nm in size (so they are also called quantum dots). High quantum efficiency, long-term photostability, narrow emission spectra, and continuous absorption spectra are some of the new characteristics of quantum dots, which are fluorescent materials used for biological labelling.
The EDC/NHS reaction can be used to conjugate biomolecules with water-soluble quantum dots functionalized with carboxyl groups (-COOH), whereas "Click Chemistry" conjugation methods can be utilized with quantum dots functionalized with azide groups (-N3). There are several surface chemistries and emission wavelengths that can be used to create these quantum dots.
Numerous biomedical applications employ quantum dots, including single protein tracking, in vivo imaging, and fluorescence assays for disease detection or drug development. For covalent conjugation with proteins, antibodies, and other biomolecules, these quantum dots are perfect. Our 550 nm quantum dots, for instance, are comparable to 6-carboxy rhodamine 6G, our 580 nm quantum dots to R-phycoerythrin (PE) or rhodamine Red-X, and our 600 nm quantum dots to Cy3.5.
Challenges and Progress in Biomedical Imaging with Quantum Dots
Fluorophores have been widely employed in biomedical imaging and detection using organic dyes. However, under typical imaging conditions, organic dyes are quickly photobleached because they are typically susceptible to the physiological environment. Additionally, they are not suitable for multicolor imaging due to their inherent characteristics. The hydrophobic surface ligands trioctylphosphine oxide (TOPO), trioctylphosphine (TOP), tetradecylphosphonic acid (TDPA), and oleic acid are typically present in quantum dots made in organic solvents.
Innovations in Water-Soluble Quantum Dots and Toxicity Mitigation
Some water-soluble bifunctional compounds that have one end that attaches to atoms on the quantum dot surface and another end that is hydrophilic and potentially reactive with biomolecules could replace these hydrophobic ligands. Toxic substances found in quantum dots include lead and cadmium (from cadmium chalcogenide-based quantum dots) (from lead chalcogenide-based quantum dots). The cells could be killed by the release of Cd2+ and Pb2+ from quantum dots. Making quantum dots well coated such that they become biologically inactive is thus a straightforward technique to prevent the potential toxicity of quantum dots. Organic polymers or compounds with minimal or no toxicity, like PEG, can be used as coating materials (e.g. ZnS and silica).
Detection of Heavy Metal Ions in Water
One of the main sources of environmental pollution is heavy metal ions. The marshal effect of heavy metal ions poses a serious threat to people, marine life, and other living things in the natural world. For the purpose of sensing heavy metal ions, a wide variety of materials have been proposed, and numerous approaches are used. Due to their toxicity and lack of biodegradability, it is necessary to be aware of them before any negative effects show. Due to their unique optical and electrical characteristics, quantum dots (QDs), which are zero-dimensional nanomaterial particles, are used as nano sensors. Broad excitation spectrum, narrow emission spectrum, and photostability are only a few of the enriched fluorescence features that QDs possess. Due to the discrete capping agents and many functional groups that are present on their surface, QDs provide diverse and sensitive detection of heavy metal ions. These capping layers and functional groups fine-tune the QDs' capacity for sensing, which makes use of the numerous ways in which QDs interact with analytes.
A significant contributor to water pollution is the presence of heavy metals as Zn, Cu, Cr, Cd, Ni, and Hg in the water. With the use of complex, expensive, and time-consuming monitoring equipment, these metal ion contaminants can be found. Quantum dots (QDs), such as CdSe, CdS, CdTe, and ZnS, among others, can be employed as quick and affordable heavy metal ion sensors as opposed to instrumentation. In different studies Zn2+ and Cu2+ ions are found in water at various concentrations and are detected using chitosan-capped ZnS QDs. To determine the presence of metal ions, the optical characteristics of the colloidal solution of ZnS are compared before and after exposure to metal ions.
Conclusion
Nanoparticles known as quantum dots are comprised of semiconducting material and have distinct optical and electrical capabilities. Water-soluble quantum dots are modified quantum dots that dissolve in water, making them simpler to work with in a variety of applications, including drug administration, biosensing, and imaging. To make these dots more stable and toxic-free, biocompatible compounds like polyethylene glycol (PEG) are typically placed on them.
To advance future technology and explore new potential realms for water soluble quantum dots, Nanografi is committed to daily efforts, bringing forth products tailored to this purpose. We invite you to explore our products offerings by visiting our website.
References
Atomically Precise Water‐Soluble Graphene Quantum Dot for Cancer Sonodynamic Therapy - Ju - 2022 - Advanced Science - Wiley Online Library. (n.d.). Retrieved January 16, 2024, from https://onlinelibrary.wiley.com/doi/10.1002/advs.202105034
Biranje, A., Azmi, N., Tiwari, A., & Chaskar, A. (2021). Quantum Dots Based Fluorescent Probe for the Selective Detection of Heavy Metal Ions. Journal of Fluorescence 2021 31:5, 31(5), 1241–1250. https://doi.org/10.1007/S10895-021-02755-8
Borgohain, R., Kumar Boruah, P., & Baruah, S. (2016). Heavy-metal ion sensor using chitosan capped ZnS quantum dots. Sensors and Actuators B: Chemical, 226, 534–539. https://doi.org/10.1016/J.SNB.2015.11.118
Graphene Quantum Dots: Properties, Synthesis & Applications - Nanografi Nano Technology. (n.d.). Retrieved January 16, 2024, from https://nanografi.com/blog/graphene-quantum-dots-properties-synthesis-applications/
Jaiswal, J. K., & Simon, S. M. (2004). Potentials and pitfalls of fluorescent quantum dots for biological imaging. Trends in Cell Biology, 14(9), 497–504. https://doi.org/10.1016/J.TCB.2004.07.012
Ju, Y.-Y., Shi, X.-X., Xu, S.-Y., Ma, X.-H., Wei, R.-J., Hou, H., Chu, C.-C., Sun, D., Liu, G., Tan, Y.-Z., Ju, Y.-Y., Ma, X.-H., Wei, R.-J., Hou, H., Tan, Y.-Z., Shi, X.-X., Xu, S.-Y., Chu, C.-C., Liu, G., & Sun, D. (2022). Atomically Precise Water-Soluble Graphene Quantum Dot for Cancer Sonodynamic Therapy. Advanced Science, 9(19), 2105034. https://doi.org/10.1002/ADVS.202105034
Larson, D. R., Zipfel, W. R., Williams, R. M., Clark, S. W., Bruchez, M. P., Wise, F. W., & Webb, W. W. (2003). Water-soluble quantum dots for multiphoton fluorescence imaging in vivo. Science, 300(5624), 1434–1436. https://doi.org/10.1126/SCIENCE.1083780/SUPPL_FILE/LARSON.SOM.PDF
Synthesis of WAGQD‐C96. a) Synthetic route of 1. i) Pd2(bda)3, Sphos,... | Download Scientific Diagram. (n.d.). Retrieved January 16, 2024, from https://www.researchgate.net/figure/Synthesis-of-WAGQD-C96-a-Synthetic-route-of-1-i-Pd2bda3-Sphos-Cs2CO3-toluene-H2O_fig1_357898413
Synthesis, Properties and Applications of Quantum Dots - Nanografi Nano Technology. (n.d.). Retrieved January 16, 2024, from https://nanografi.com/blog/synthesis-properties-and-applications-of-quantum-dots/
Water-Soluble Quantum Dots for Multiphoton Fluorescence Imaging in Vivo | Science. (n.d.). Retrieved January 16, 2024, from https://www.science.org/doi/10.1126/science.1083780
Water Soluble Quantum Dots – NanoOptical Materials. (n.d.). Retrieved January 16, 2024, from https://nomcorp.com/water-soluble-quantum-dots/
Water-soluble quantum dots – Reagents and equipment for R&D of lateral flow assays. (n.d.). Retrieved January 16, 2024, from https://www.lateralflows.com/product/water-soluble-quantum-dots/
Water-Soluble Quantum Dots for Multiphoton Fluorescence Imaging in Vivo | Science. (n.d.). Retrieved January 16, 2024, from https://www.science.org/doi/10.1126/science.1083780
Recent Posts
-
MXenes from MAX Phases
MXenes, a group of two-dimensional materials derived from MAX phases, are gaining significant tracti …19th Oct 2024 -
Preserving History with Graphene's Power
Cultural artifacts are at risk of deterioration over time due to the destructive effects of both na …11th Oct 2024 -
The Role of Graphene in Neuroelectronics
Neuroelectronics is an interdisciplinary field that aims to develop devices that can interact with t …4th Oct 2024






