MXenes from MAX Phases
MXenes, a group of two-dimensional materials derived from MAX phases, are gaining significant traction in advanced technological applications such as energy storage, sensors, and water purification. Their unique structure, surface chemistry, and tunable properties make them versatile materials for a variety of uses.
This blog examines the synthesis process of MXenes from MAX phases, their characterization methods, and the ways they are revolutionizing the fields they are applied to. By offering high-quality MAX phase materials, Nanografi empowers researchers and industries to explore the full potential of MXenes in advanced applications such as energy storage, sensors, and water purification.
Introduction
In the field of two-dimensional materials, the discovery of MXenes has marked a significant breakthrough. Since their introduction in 2011, MXenes have been recognized for their exceptional chemical, mechanical, and electrical properties. These materials are synthesized from layered MAX phases, consisting of transition metals, A-group elements, and carbon or nitrogen. Through a selective etching process, the A-group layers are removed, leaving behind the transition metal carbide or nitride layers, which form the MXene structure.
What are MAX Phases?
MAX phases are ternary compounds with the general formula Mn+1AXn, where M stands for an early transition metal (such as titanium or vanadium), A is an element from groups 13 or 14 (like aluminum or silicon), and X represents carbon and/or nitrogen. These materials are known for their unique combination of metallic and ceramic properties, exhibiting electrical conductivity similar to metals while maintaining the strength and resistance typical of ceramics. MAX phases are layered structures, which makes them an ideal precursor for producing MXenes through selective etching processes.
The layered structure of MAX phases involves alternating layers of M-X units, separated by A-group elements. The key to creating MXenes is to selectively remove these A-group elements, freeing the M-X layers and giving rise to the unique properties of MXenes. Common MAX phases used for MXene synthesis include Ti3AlC2, Ti2AlC, and V2AlC.
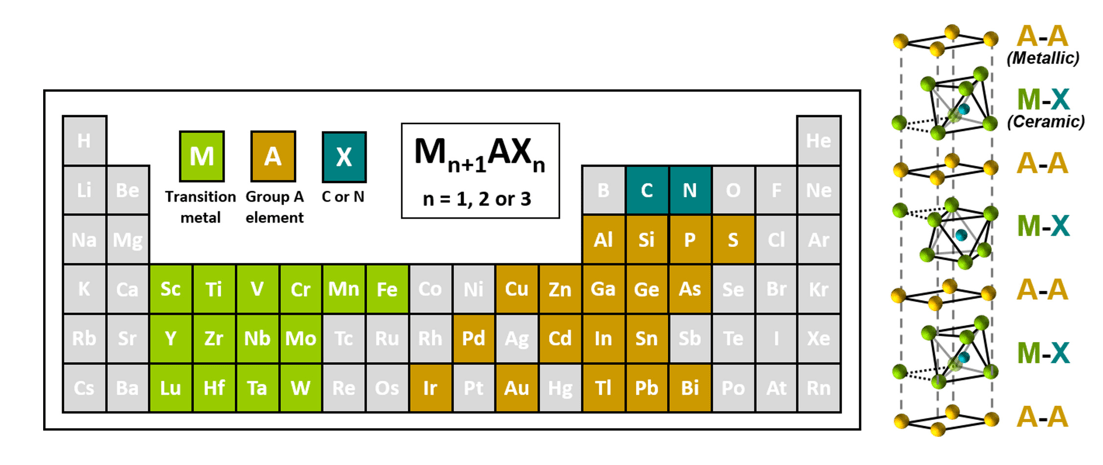
Figure 1. The elements from the periodic table present in MAX phases and their unit cell structure.
What are MXenes?
MXenes are two-dimensional materials obtained by selectively etching the A-group elements from MAX phases. They retain the layered structure of their parent MAX phases but exhibit different properties due to the absence of the A-element and the presence of surface terminations like hydroxyl (OH), oxygen (O), or fluoride (F). These surface terminations play a crucial role in determining the properties of MXenes, including their hydrophilicity, electrical conductivity, and chemical reactivity.
MXenes have shown tremendous versatility in various applications, ranging from energy storage devices like batteries and supercapacitors to water purification systems. Their high electrical conductivity, combined with their ability to be easily functionalized, makes them suitable for applications that require both high-performance materials and flexibility in chemical composition. MXenes' two-dimensional structure also offers a high surface area, which is advantageous in applications such as catalysis and electromagnetic interference (EMI) shielding.
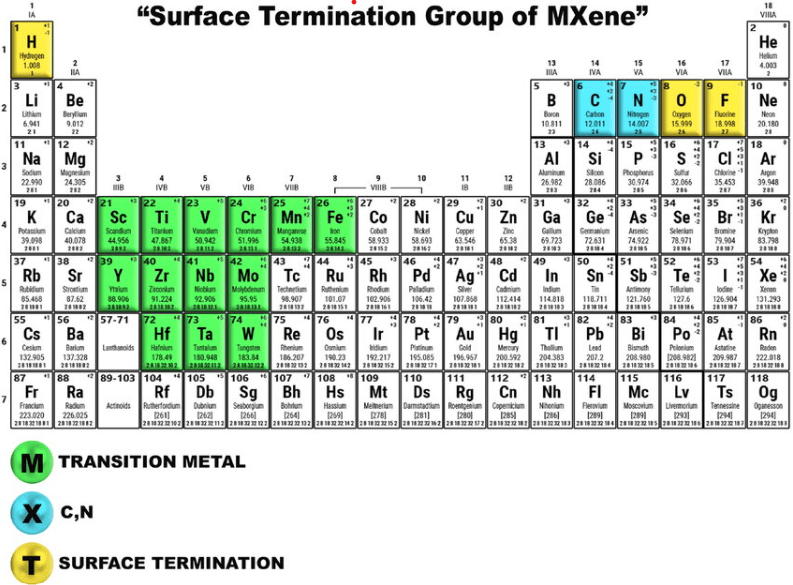
Figure 2. Schematic representation of the surface termination groups of MXene.
Synthesis of MXenes from MAX Phases
The most common method for synthesizing MXenes involves selective etching of the A-group element from MAX phases using fluoride-containing acids, typically hydrofluoric acid (HF). During this process, the MAX phase powder is slowly added to an HF solution, which removes the A-group layers and leaves behind the transition metal carbide or nitride layers in the form of MXenes. After etching, the mixture is washed and filtered to obtain multi-layered MXene flakes, which can be further processed to produce single- or few-layer MXenes through delamination.
An alternative to HF etching is the use of a mixture of fluoride salts with hydrochloric acid (HCl), which can produce MXenes without the safety concerns associated with HF. Additionally, molten salt etching has emerged as a novel method for synthesizing MXenes,offering better control over the etching process and reducing the risk of structural damage to the MXene layers.
Key parameters that influence the quality of synthesized MXenes include the concentration of the etching solution, etching time, and temperature. Longer etching times and higher temperatures can lead to more efficient removal of A-group elements but may also result in defects or the collapse of the MXene structure. Therefore, careful control of the synthesis parameters is crucial to obtaining high-quality MXenes.
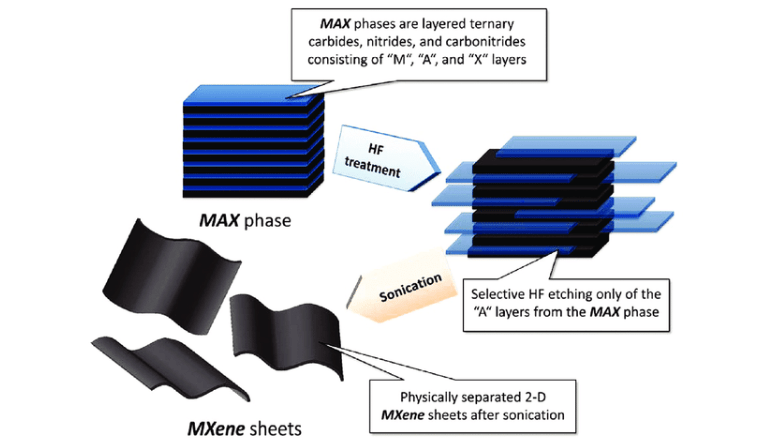
Figure 3. Etching process of the MAX phase to MXene nanosheets.
Characterization Techniques for MXenes
Various characterization methods are employed to examine the structural and chemical properties of MXenes. These techniques provide critical insights into the material's composition, surface terminations, and overall quality.
Electron Microscopy
Electron microscopy plays a pivotal role in characterizing MXenes, providing detailed insights into their structure and composition.
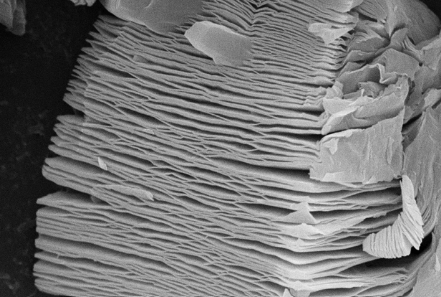
- Scanning Electron Microscopy (SEM): SEM is commonly used to confirm the layered structure of MXenes and to verify the successful removal of A-group elements from the MAX phases. SEM images reveal the characteristic two-dimensional morphology of MXenes, showing the separation of the layers after etching.
- Transmission Electron Microscopy (TEM): TEM allows for high-resolution imaging of the MXene structure at the atomic level. It is particularly useful for visualizing the lattice fringes and determining the degree of crystallinity. TEM also provides valuable information on the thickness and uniformity of MXene layers, making it an essential tool for assessing the quality of the synthesized material.
Spectroscopic Analysis
Spectroscopic techniques are crucial for analyzing the surface chemistry and elemental composition of MXenes.
- X-ray Photoelectron Spectroscopy (XPS): XPS is used to identify the surface terminations present on MXenes, such as hydroxyl (OH), oxygen (O), or fluoride (F). These terminations significantly influence the chemical reactivity and electrical properties of MXenes. XPS provides detailed information on the oxidation states of the elements present and helps to confirm the successful etching of the A-group elements.
- Energy Dispersive X-ray Spectroscopy (EDX): Often coupled with SEM or TEM, EDX is used to determine the elemental composition of MXenes. It confirms the presence of transition metals and carbon/nitrogen while ensuring that the A-group elements have been successfully removed.
Figure 4. SEM image of Nanografi's Titanium Carbide (Ti3C2Tx) MXene Phase Powder product. Review product.
MXene Application Areas
MXenes, one of the two-dimensional materials derived from MAX phases, have the potential for wide use in advanced technological applications such as energy storage, sensors and water treatment.
Energy Storage
MXenes play an important role in energy storage devices, especially batteries and supercapacitors. Thanks to their high electrical conductivity and large surface area, MXenes are effective in increasing the energy storage capacity and improving the charge and discharge rates of devices.
Compared to conventional battery materials, MXenes support fast charge/discharge cycles and offer longer cycle life. Furthermore, since the surface chemistry of MXenes is customisable, they can be adapted to different energy storage requirements. This helps optimise the energy density and power density of batteries.
Sensors
MXenes also have a wide range of applications in environmental, biological and chemical sensors.The high surface area and fine structure of MXenes allow fast and sensitive interaction with small molecules.Especially in gas sensing, biosensors and humidity sensors, MXenes offer high sensitivity and fast response times.
The customisability of their electrical and chemical properties allows them to be optimised for different sensor applications.For example, a surface coated with MXenes can measure the change in conductivity when interacting with target molecules, increasing the sensitivity of sensors.
Water Treatment
MXenes also have great potential in water treatment systems. High surface areas and customisability of surface terminations make MXenes effective in filtration and adsorption processes. MXenes can provide effective removal of heavy metals, organic pollutants and ions in water.
Thanks to their conductive structure, they can also be used in electrochemical water treatment methods. In addition, MXenes can also be used as membranes in techniques such as nanofiltration and reverse osmosis, thereby accelerating processes such as water purification, desalination and elimination of harmful substances.
Conclusion
MXenes are poised to revolutionize a variety of industries with their unique properties and versatile applications. Their synthesis from MAX phases, combined with advanced characterization techniques, has enabled researchers to explore their full potential. As research continues, MXenes are expected to play a key role in the development of future technologies, from energy storage to environmental protection.
To follow the latest developments and innovations related to nanotechnology, visit Blografi.
References
Alnoor, H., Elsukova, A., Palisaitis, J., Persson, I., Tseng, E. N., Lu, J., Hultman, L., & Persson, P. O. Å. (2021). Exploring MXenes and their MAX phase precursors by electron microscopy. Materials Today Advances, 9, 100123. https://doi.org/10.1016/J.MTADV.2020.100123
Ghosh, A., Pal, H., Das, T., Chatterjee, S., & Das, A. (2022). Synthesis and Characterization of MXene from MAX phase. Materials Today: Proceedings, 58, 714–716. https://doi.org/10.1016/J.MATPR.2022.02.253
Gonzalez-Julian, J. (2021). Processing of MAX phases: From synthesis to applications. Journal of the American Ceramic Society, 104(2), 659–690. https://doi.org/10.1111/JACE.17544
Koneru, B., Swapnalin, J., Pothu, R., Banerjee, P., Boddula, R., Radwan, A. B., & Al-Qahtani, N. (2023). Role of the intercalated ions on the high capacitance behavior of Ti 3 C 2 Tx MXene nanohybrids. Nanocomposites, 9(1), 128–137. https://doi.org/10.1080/20550324.2023.2258622
Kruger, D. D., García, H., & Primo, A. (2024). Molten Salt Derived MXenes: Synthesis and Applications. Advanced Science, 11(35), 2307106. https://doi.org/10.1002/ADVS.202307106
Recent Posts
-
MXenes from MAX Phases
MXenes, a group of two-dimensional materials derived from MAX phases, are gaining significant tracti …19th Oct 2024 -
Preserving History with Graphene's Power
Cultural artifacts are at risk of deterioration over time due to the destructive effects of both na …11th Oct 2024 -
The Role of Graphene in Neuroelectronics
Neuroelectronics is an interdisciplinary field that aims to develop devices that can interact with t …4th Oct 2024