What is the Role of Nanomaterials in Catalysis?
Catalysis is a fundamental process in chemistry that accelerates chemical reactions by providing an alternative reaction pathway with a lower activation energy. The introduction of nanomaterials into the field of catalysis has significantly advanced this discipline, leveraging the unique properties of nanoparticles to enhance catalytic efficiency and selectivity.
This article comprehensively examines the principles of catalysis, its historical development, and the innovative applications of various nanomaterials in improving catalytic performance. Nanografi provides the production and supply of all elements in the periodic table in nano and micron sizes. Discover Nanografi now.
Introduction
Nanomaterials used in catalysis, such as metal nanoparticles, carbon-based nanostructures, and metal oxides represent a cutting-edge approach to enhancing the efficiency and speed of chemical reactions. These materials, by virtue of their high surface area and unique electronic properties, play a pivotal role in a wide range of applications including energy production, environmental remediation, and fine chemical synthesis. The development of advanced nanomaterials continues to drive significant progress in catalytic processes, leading to more sustainable and effective solutions.
What is Catalysis?
Imagine you are trying to push a heavy box up a hill. It takes a lot of effort and energy to get it moving. Now, imagine someone places a ramp on the hill. With the ramp, you can push the box up more easily and with less effort. In this analogy, the ramp is like a catalyst.
In chemistry, catalysis is a process where a substance called a catalyst helps speed up a chemical reaction. Just like the ramp helps you push the box more easily, a catalyst makes it easier for a reaction to happen. Importantly, the catalyst itself doesn’t get used up in the reaction – it just helps things along.
In brief, a catalyst is a substance that increases the rate of a chemical reaction by providing an alternative reaction pathway with a lower activation energy, without being consumed in the process. In essence, a catalyst facilitates the transformation of reactants into products more efficiently.
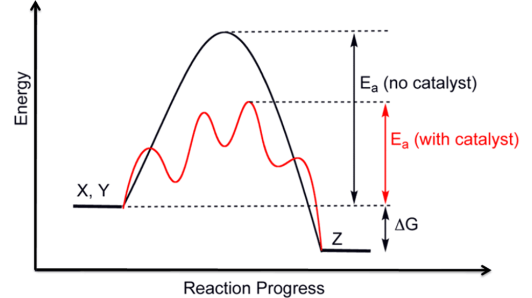
Figure 1. The catalysis increases the rate of a reaction by lowering its activation energy.
Historical Background of Catalysis
The employment of nanoparticles in catalysis is rooted in antiquity, with gold nanoparticles being used in ancient China and Egypt for both medicinal and decorative purposes, reflecting their remarkable properties. The scientific exploration of nanoparticle catalysis gained significant momentum in the 17th century, with early treatises on colloidal nanoparticles laying the foundation. A pivotal moment occurred in the mid-19th century when Michael Faraday's work elucidated the optical properties of colloidal gold. The 20th century witnessed substantial progress, particularly with the utilization of palladium and platinum nanoparticles in hydrogenation reactions.
A discovery by Haruta in 1987 demonstrated that gold nanoparticles smaller than 5 nm exhibit extraordinary catalytic activity for carbon monoxide oxidation, underscoring the critical impact of nanoparticle size on catalytic performance. These historical milestones have paved the way for contemporary research in nanocatalysis, which continues to evolve and expand its scope.
How Catalysts Work?
Catalysts are substances that increase the rate of a chemical reaction without being consumed in the process. They function by providing an alternative reaction pathway with a lower activation energy.
Here is a detailed explanation of how catalysts work:
1. Lowering Activation Energy
Every chemical reaction involves an energy barrier that must be overcome for reactants to transform into products. This energy barrier is known as the activation energy. Catalysts lower the activation energy required for the reaction, making it easier for reactants to convert into product.
2. Formation of Intermediates
Catalysts often participate in the formation of transient intermediates. These intermediates are more reactive than the original reactants and can quickly convert into the final products. The catalysts is regenerated at the end of the reaction cycle, ready to facilitate another reaction.
3. Surface Catalysis
In heterogeneous catalysis, the catalysts is typically a solid, and the reactants are in a different phase (usually gases or liquids). The reactants adsorb onto the catalyst's surface, where the reaction occurs. The products then desorb from the surface, leaving the catalyst unchanged. This process includes:
- Adsorption: Reactants bind to the catalyst's surface.
- Reaction: Reactants interact on the surface, leading to the formation of products.
- Desorption: Products leave the catalysis's surface.
4. Enzyme Catalysis
In biological systems, enzymes act as catalysts. They have highly specific active sites where reactants, known as substrates, bind. The enzyme-substrate complex lowers the activation energy, facilitating the conversion of substrates into products. Enzymes are highly selective and can catalyze reactions under mild conditions.
5. Homogeneous Catalysis
In homogeneous catalysis, the catalyst and reactants are in the same phase, usually a liquid. The catalyst forms an intermediate complex with the reactants, which then undergoes a reaction to form the products. The catalysis is released in its original form at the end of the reaction.
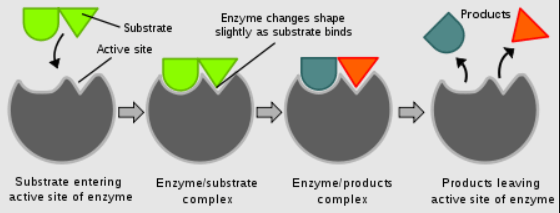
Figure 2. Schematic representation of the effect of catalysis.
Key Characteristics of Catalysis:
- Specificity: Catalysts are often specific to particular reactions.
- Regeneration: Catalysts are not consumed in the reaction and can be used repeatedly.
- Selectivity: Catalysts can influence the formation of particular products over others.
- Efficiency: Catalysts significantly increase the rate of reaction, often by many orders of magnitude.
Use of Nanomaterials in Catalysis
Nanomaterials provide unique properties that make them excellent catalyst. The small size of nanoparticles allows for a greater proportion of atoms to be available on the surface, increasing the active sites for catalysis and thus boosting reaction rates and overall efficiency.
Additionally, the electronic properties of nanoparticles can be tuned by altering their size, shape, and composition, optimizing catalytic activity and selectivity. Modern applications of nanocatalysis include CO2 capture, hydrogen production, and environmental remediation.
Types of Nanomaterials in Catalysis
There are various types of nanomaterials, each offering distinct advantages for different catalytic applications:
Metal Nanoparticles
Metal nanoparticles, such as platinum, palladium, and gold, are widely studied for their catalytic properties in reactions like hydrogenation, oxidation, and coupling reactions. These nanoparticles are highly effective due to their ability to facilitate reactions under milder conditions and their high selectivity.
Metal nanoparticles can be dispersed on support materials, maximizing the surface area and active sites available for reactions. Additionally, alloying different metals at the nanoscale creates alloyed nanoparticles with enhanced catalytic properties, such as combining platinum with palladium to improve both activity and stability.
Metal Oxide Nanoparticles
Metal oxides like titanium dioxide, zinc oxide, and cerium oxide serve as both catalysts and supports for metal nanoparticles. They are crucial in reactions like photocatalysis and environmental remediation due to their stability and reactivity. For instance, platinum nanoparticles supported on titanium dioxide (TiO2) enhance photocatalytic activity for environmental cleanup processes.
Core-shell structures, where one type of nanoparticle forms the core and another material forms the shell, are used to protect the core material and enhance overall catalytic efficiency, such as using a gold core with a platinum shell for improved performance in fuel cells.
Carbon-Based Nanomaterials
Carbon-based nanomaterials, including carbon nanotubes, graphene, and fullerenes, offer high electrical conductivity, large surface area, and chemical stability. These properties make them suitable for various catalytic processes, including electrocatalysis and heterogeneous catalysis.
Functionalizing carbon nanotubes with acidic groups can improve their performance in specific catalytic reactions. Additionally, integrating active nanocatalysts with semiconductors or plasmonic particles can facilitate photo-induced catalytic reactions, such as using carbon-based materials in solar-to-fuel energy conversion.
Conclusion
Nanomaterials offer innovative solutions such as enhanced photocatalytic and electrocatalytic processes, which are crucial for renewable energy technologies. The development of composite nanomaterials and the strategic design of nanostructures provide superior catalytic activity and stability, addressing both current industrial challenges and future technological needs. As research progresses, the synergetic collaboration between academia and industry will be essential in unlocking the full potential of nanocatalysts, paving the way for groundbreaking applications and more sustainable chemical processes.
To follow the latest developments and innovations related to nanotechnology, visit Blografi.
References
Khalil, M., Kadja, G. T. M., & Ilmi, M. M. (2021). Advanced nanomaterials for catalysis: Current progress in fine chemical synthesis, hydrocarbon processing, and renewable energy. Journal of Industrial and Engineering Chemistry, 93, 78–100. https://doi.org/10.1016/J.JIEC.2020.09.028
Michael Faraday’s gold colloids | Royal Institution. (n.d.). Retrieved August 5, 2024, from https://www.rigb.org/explore-science/explore/collection/michael-faradays-gold-colloids
Okumura, M., Fujitani, T., Huang, J., & Ishida, T. (2015). A Career in Catalysis: Masatake Haruta. ACS Catalysis, 5(8), 4699–4707. https://doi.org/10.1021/acscatal.5b01122
Retrieved August 5, 2024, from https://ch302.cm.utexas.edu/kinetics/catalysts/catalysts-all.php
What Is a Catalyst? Understand Catalysis. (n.d.). Retrieved August 5, 2024, from https://sciencenotes.org/what-is-a-catalyst-understand-catalysis/
Yentekakis, I. V., Gournis, D. P., & Karakassides, M. A. (2023). Nanomaterials in Catalysis Applications. Catalysts 2023, Vol. 13, Page 627, 13(3), 627. https://doi.org/10.3390/CATAL13030627
Recent Posts
-
Turning Noise into Power: Energy Harvesting with Piezoelectric Nanogenerators
Ambient acoustic energy, once an untapped resource, is now being converted into sustainable electric …5th Mar 2025 -
Holey Super Graphene in Li-ion Batteries: Next Generation of Energy Storage
Holey Super Graphene (hG), also referred to as “holey graphene,” is redefining li-ion ba …7th Feb 2025 -
Future Communication with 5G Technology and Advanced Materials
5G technology opens the doors to a new era in communication with faster connection speeds, low laten …6th Feb 2025