Polytetrafluoroethylene as Excellent Binder for Li-ion Battery
It is Essential for lithium-ion batteries to have an appropriate binder for their electrodes. In fact, the binders must have enough durability and a long life as significant requirements to be employed for electrodes.
Basically, binders need to be electrochemically stable and chemically inert in order for lithium-ion batteries to show excellent performance. Furthermore, PTFE condensed liquid binders show the best performance via the use of smaller amounts of them on electrodes surface. Nanografi offers high-quality PTFE which can be crucial for researchers and industries aiming to enhance the performance and longevity of lithium-ion batteries through superior binding solutions.
Introduction
Among the most common binders for applications in electrode coverings in lithium-ion batteries, polytetrafluoroethylene condensed liquid binder has shown promising results when employed as the binder agent. As a synthetic fluoropolymer, PTFE has a high molecular weight comprising carbon and fluorine atoms and is ranked as a hydrophobic material making it inert and not attractive when placed in water or in contact with aqueous solutions more specifically because of the London forces coming from the high electronegativity of fluorine.
Binding Properties of Polytetrafluoroethylene
Technically speaking, PTFE binders contain units of CF2-CF2 demonstrating desirable chemical stability partly being used in lithium-ion batteries. PTFE condensed liquid binder, on the other hand, is known as an environmentally friendly agent without any hazardous effect in the atmosphere in which water is employed as a solvent with no harmful and health threats. Another advantage of PTFE is its excellent chemical resistance in addition to its heat and light and cold resistance. PTFE also provides desirable fire resistance and lower water absorption as well as resistance against ultraviolet radiation with excellent performance in extreme weather conditions.
Polytetrafluoroethylene (PTFE)
Polytetrafluoroethylene is originally a synthetic fluoropolymer of tetrafluoroethylene with a lot of applications discovered in the 1930s with a brand name of Teflon. PTFE is a solid of fluorocarbon with a high molecular weight composed of basically carbon and fluorine with a hydrophobic behavior in which water or any water-containing substance can interact doesn’t interact with it. Fluorocarbons have appeared to show improved London dispersion forces because of their practically high electronegativity of fluorine with low cohesion fraction among solids. PTFE has broad applications as a coating for cooking pans and other cookware as a non-reactive agent. The inert nature of PTFE is partly due to their strong carbon and fluoride bonds making it a qualified agent for coating containers and pipework for reactive and corrosive chemicals. When PTFE is employed as a lubricant, it reduces the friction, energy consumption of machinery and the wear on the surfaces. It also has common applications as graft material and surgical interventions. PTFE has been frequently employed in medical devices such as catheters to adhere to bacteria and germs and stop hospital-acquired infections.
PTFE is a thermoplastic polymer seeming like a white solid material at room temperature whose density is nearly 2,200 kilogram per cubic meter. Based on research, it has a melting point of about 600 Kelvin capable of maintaining high self-lubrication, strength and toughness at lower temperatures of nearly -268°C. At temperatures of about 194 Kelvin, PTFE manifests excellent flexibility. In terms of its inertness against chemicals, it should be noted that the chemical carbon-fluorine bonds of PTFE seem to be affected by highly reactive metals like alkali metals at higher temperatures as well as metals including magnesium and aluminium along with some fluorinating agents namely Cobalt fluoride and Xenon difluoride. Another important point concerning the properties of PTFE is it undergoes depolymerization at temperatures above 650°C.
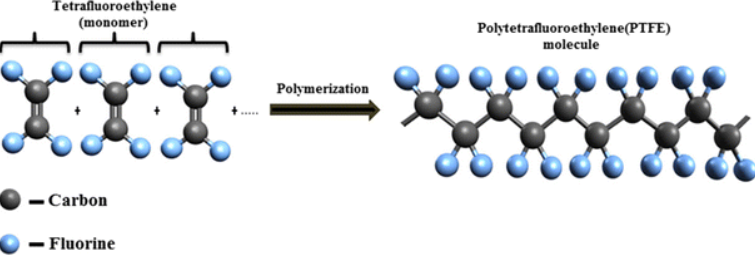
Figure 1. Structure of of PTFE.
Li-Ion Batteries
Lithium batteries are deemed to be the main source of power or backup power source for portable communication devices as well as mobile electronic devices. Lithium batteries have been calling increasing attention in the industrial and scientific sectors because of their remarkable electromotive force and high energy density. Recently, there has been a huge amount of research in order to design novel structures of electrode materials and develop never-before-seen battery binders so that the demand for higher energy density is mitigated and cycle properties of batteries are met. In effect, aqueous binders have shown to possess more advantages properties and merits including lower-cost, battery safety and friendliness ecosystem in combination with water as a dispersant to make them emerge as the ideal binders for environmentally friendly lithium-ion batteries and safety.
To learn more about lithium-ion battery materials, you can read our blog post here.
Self-Binding Composite Separator Based on Poly (tetrafluoroethylene) Coating for Li-Ion Batteries
A newly published study suggests the application of a novel composite separator composed of polytetrafluoroethylene PTFE coating layers and its commercial polyethylene separator developed for designing high-performance Lithium-ion batteries. This composite separator is fabricated by immersing a PE separator into a suspension containing PTFE in order to obtain a self-binding PTFE/PE/PTFE structure composed of three layers. In this is, the as-prepared composite separator is modified further with hydrogen peroxide and sulfuric acid solution in order to increase its electrolyte affinity. Based on the results, the coating layer made of close-packed PTFE particles adopts a highly ordered nanoporous structure with the highly desirable electrolyte wettability characteristic. This greatly increase the ionic conductivity of the composite separator which manifests desirable thermal stability when compared to polyethylene separator alone reaching the thermal resistance grade of commercial separators coated by ceramics. CR2032 type unit half cells composed of the LiFePO4 cathode and lithium anode are assembled using PTFE based separators whose carbon rate and cycling performances show promising results after evaluations. The assembled cells based on the composite separator has been showing to adopt a better Carbon rate capability and cycling capacity retention with polyethylene separator alone. It is interesting to be mentioned that the composite separator could be employed as a potential candidate for coating purposes in high-performance rechargeable Lithium-ion batteries.2
Conclusion
Lithium-ion batteries are extensively used in power source fields including electric vehicles, power tools and electronic devices. Up to this point, different kinds of high-performance cathode and anode materials have been employed in order to take care of the growing and increasing demand for high power and high rate batteries. Separators and coverings are crucial for improving the performance of electronic products more specifically, lithium-ion batteries, supercapacitors and so forth. Polypropylene and polyethylene make critical components of lithium-ion batteries but with poor wettability and thermal stability causing serious concerns about the safety of Lithium-ion batteries when there is a probability of unusual heat generation. These failures greatly contribute to the use of PTFE as a Condensed liquid binder in Li-ion batteries.
To discover the latest news from nanotechnology, you can visit Blografi.
References
From Graphene to the New Teflon - Nanografi Nano Technology. (n.d.). Retrieved April 19, 2024, from https://nanografi.com/blog/from-graphene-to-the-new-teflon/
Lithium Lanthanum Zirconate in Lithium-Ion Battery Applications - Nanografi Nano Technology. (n.d.). Retrieved April 19, 2024, from https://nanografi.com/blog/lithium-lanthanum-zirconate-in-lithiumion-battery-applications/
Lithium-Ion Batteries: How They Work, Where They Are Used, Advantages & Disadvantages - Nanografi Nano Technology. (n.d.). Retrieved April 19, 2024, from https://nanografi.com/blog/lithiumion-batteries-how-they-work-where-they-are-used-advantages-disadvantages/
Molecular structure of polytetrafluoroethylene (PTFE) | Download Scientific Diagram. (n.d.). Retrieved April 19, 2024, from https://www.researchgate.net/figure/Molecular-structure-of-polytetrafluoroethylene-PTFE_fig2_323372687
Online, V. A. batteries †. 24859–24862 (2014) doi:10.1039/c4ra01351d.
Zhang, K., Xiao, W., Liu, J. & Yan, C. A Novel Self-Binding Composite Separator Based on. 1–13 (2018) doi:10.3390/polym10121409.
Recent Posts
-
Turning Noise into Power: Energy Harvesting with Piezoelectric Nanogenerators
Ambient acoustic energy, once an untapped resource, is now being converted into sustainable electric …5th Mar 2025 -
Holey Super Graphene in Li-ion Batteries: Next Generation of Energy Storage
Holey Super Graphene (hG), also referred to as “holey graphene,” is redefining li-ion ba …7th Feb 2025 -
Future Communication with 5G Technology and Advanced Materials
5G technology opens the doors to a new era in communication with faster connection speeds, low laten …6th Feb 2025






