What are Lead Sulfide Quantum Dots (PbS QDs)?
Lead sulfide (PbS) quantum dots represent a significant advancement in the field of nanotechnology, offering innovative solutions to various technological challenges.
These quantum dots exhibit distinct properties attributable to their nanoscale dimensions, rendering them suitable for a wide range of applications, including photovoltaics, biological imaging, and optoelectronics. By delving into the specifics of PbS quantum dots, from their synthesis and unique properties to their diverse applications, we can appreciate their transformative impact on multiple industries. By integrating lead sulfide quantum dots, Nanografi aims to contribute to the advancement of high-efficiency, flexible, and cost-effective photovoltaic solutions.
Introduction
Lead sulfide (PbS) quantum dots (QDs) are semiconductor nanocrystals that exhibit unique optical and electronic properties due to their nanoscale dimensions. This guide explores their synthesis, properties, and applications, highlighting their significance in modern technology.
Understanding Lead Sulfide Quantum Dots (PbS QDs)
Lead Sulfide Quantum Dots (PbS QDs) are nanoscale semiconductor particles made from lead sulfide (PbS). They possess unique optical and electronic properties due to their small size, typically ranging from 2 to 10 nanometers in diameter. The quantum dots' size is crucial as it is smaller than the Bohr exciton radius, this property allows them to absorb and emit light at specific wavelengths, making them highly valuable in various applications, including photovoltaics, biological imaging, and optoelectronics.
Properties of Lead Sulfide Quantum Dots (PbS QDs)
Lead sulfide (PbS) quantum dots have properties such as tunable optical properties, high stability, and significant infrared interaction, making them valuable for a variety of advanced technological applications.
Quantum Confinement Effect
Due to their small size, PbS QDs exhibit discrete energy levels rather than continuous bands. This confinement leads to unique optical properties, such as size-tunable bandgaps, where the energy gap between the conduction and valence bands changes with the size of the quantum dots. Smaller dots have larger bandgaps, leading to the emission of light at shorter wavelengths, while larger dots emit at longer wavelengths.
To get all the information about quantum dots, read our blog post.
Size-Tunable Properties
The optical absorption and photoluminescence (light emission) properties of PbS QDs can be precisely controlled by adjusting their size. This tunability makes them suitable for applications requiring specific wavelengths of light, such as infrared detectors, solar cells, and biomedical imaging.
Infrared Spectrum
Lead sulfide (PbS) quantum dots are notable for their ability to interact with the infrared (IR) spectrum. Infrared light is a type of electromagnetic radiation with longer wavelengths than visible light, which makes it invisible to the human eye. However, many technologies rely on detecting and using infrared light, such as night-vision cameras, remote controls, and certain types of sensors.
Why Infrared Matters?
Infrared light is often used in applications where it is important to see or detect things that are not visible to the naked eye. For example, infrared cameras can capture heat images, allowing us to see the temperature differences in objects, which is useful in medical imaging and thermal inspections.
Lead sulfide quantum dots are particularly effective in the infrared range because of their unique electronic properties. When these quantum dots are exposed to light, they can absorb and emit it in the infrared spectrum. This makes them highly valuable for creating devices that need to detect or use infrared light.
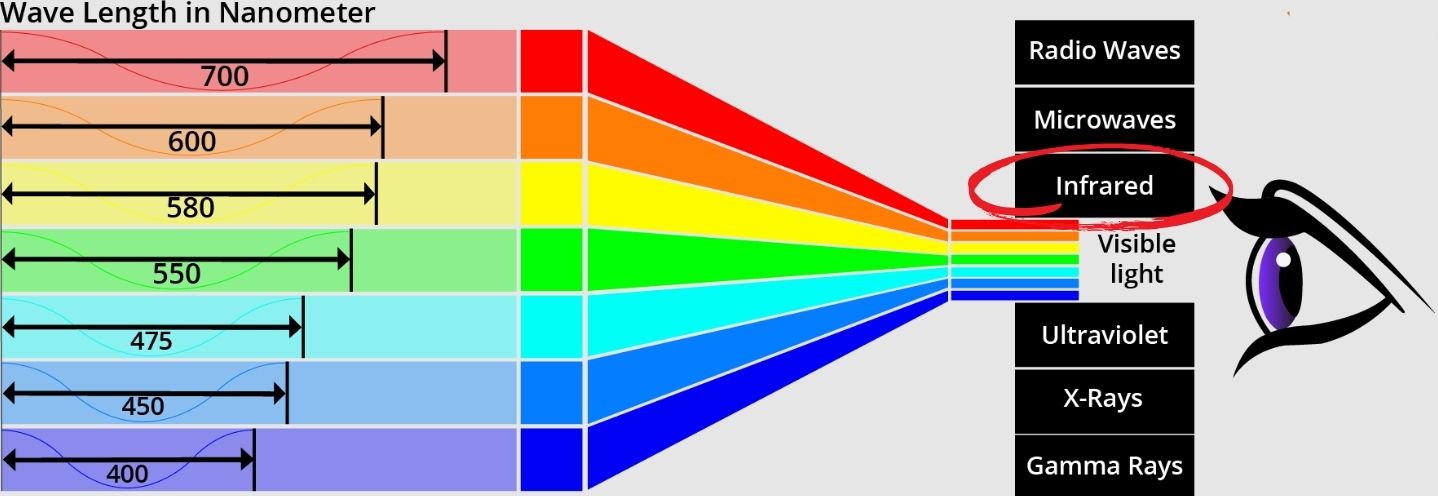
Figure 1. Illustration of color spectrum wavelength.
Photostability
PbS QDs are photostable, meaning they do not degrade easily under light exposure. This stability is crucial for applications in long-term imaging and sensing.
Surface Chemistry
The surface of PbS QDs can be modified with various ligands to improve their
solubility, stability, and biocompatibility. Surface modification also allows
for the integration of quantum dots into different matrices and the creation of
hybrid materials with tailored properties.
Electronic Properties
- High Carrier Mobility: PbS QDs have high electron and hole mobility, making them suitable for electronic and optoelectronic applications.
- Quantum Confinement Effect: Due to their small size, the electrons and holes are confined in a potential well, leading to discrete energy levels rather than continuous bands.
Stability
- Chemical Stability: PbS QDs can be sensitive to oxidation, requiring surface passivation or encapsulation to maintain stability.
- Thermal Stability: PbS QDs generally exhibit good thermal stability, but their properties can be affected by high-temperature treatments.
Toxicity and Environmental Impact
The presence of lead in PbS QDs raises concerns about toxicity and environmental impact. Proper handling, disposal, and potential substitution with less toxic materials are important considerations.
Synthesis of Lead Sulfide Quantum Dots (PbS QDs)
Lead sulfide quantum dots' synthesis involves controlling the size and shape of these nanocrystals to achieve desired properties for various applications, such as in photovoltaics, sensors, and biological imaging. Several methods are available for synthesizing PbS QDs, including chemical, physical, and biological approaches.
Chemical Synthesis
Chemical synthesis is the most commonly used method for producing PbS QDs due to its precision and scalability. The key steps involve:
- Preparation of Precursors: Lead and sulfur precursors are prepared. Typically, lead acetate (Pb(CH₃COO)₂) or lead oxide (PbO) is used as the lead source, while thioacetamide (CH₃CSNH₂) or sodium sulfide (Na₂S) is used as the sulfur source.
- Reaction Environment: The reaction is conducted in a controlled environment, often in an inert atmosphere like nitrogen or argon, to prevent oxidation.
- Temperature Control: The reaction mixture is heated to a specific temperature, usually between 80°C and 150°C, to facilitate nucleation and growth of the quantum dots.
- Capping Agents: Organic ligands or capping agents such as oleic acid, trioctylphosphine oxide (TOPO), or mercaptopropionic acid are added to stabilize the quantum dots and control their size.
- Purification: The synthesized QDs are purified by precipitation and redispersion in solvents like ethanol or hexane to remove unreacted precursors and by-products.
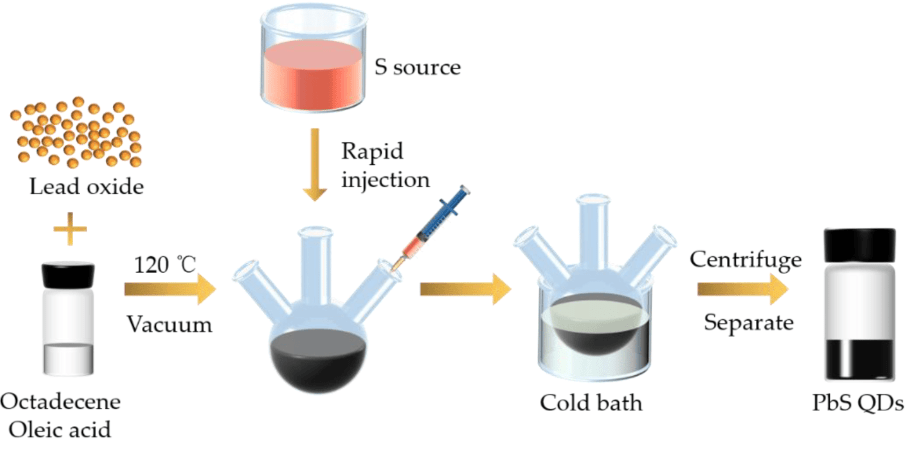
Figure 2. The fundamental schematic for the preparation of PbS quantum dots (PbS QDs).
Physical Synthesis
Physical methods, including laser ablation and sputtering, are less common but offer certain advantages:
- Laser Ablation: A bulk lead sulfide target is ablated using a high-power laser in a liquid medium. The ablation process creates PbS nanoparticles that are then stabilized using surfactants.
- Sputtering: PbS films are deposited onto a substrate using a sputtering process, followed by annealing to form QDs.
Biological Synthesis
Biological synthesis is an eco-friendly approach that uses biological molecules as reducing and stabilizing agents:
- Biomolecules: Plant extracts, bacteria, or fungi can act as reducing agents to convert lead ions into PbS nanoparticles.
- Green Chemistry: This method reduces the need for toxic chemicals and operates under mild conditions, making it environmentally benign.
Applications of Lead Sulfide Quantum Dots (PbS QDs)
Lead sulfide quantum dots (PbS QDs) are utilized in various applications such as photovoltaics, sensors, optoelectronics, catalysis, and biological imaging due to their unique optical and electronic properties.
Photovoltaics
Lead Sulfide Quantum Dots are utilized in the development of advanced solar cells. Due to their size-tunable bandgap and high absorption coefficients in the near-infrared (NIR) region, they are particularly effective in capturing a broad spectrum of sunlight. This enhances the efficiency of solar cells, especially in tandem configurations where PbS QDs can complement other materials that absorb different parts of the solar spectrum.
Photodetectors
PbS QDs are also used in photodetectors, which are devices that sense light and convert it into an electrical signal. Their sensitivity to NIR light makes them suitable for applications in telecommunications, imaging, and environmental monitoring. PbS QD photodetectors offer high sensitivity and fast response times, making them ideal for high-performance applications.
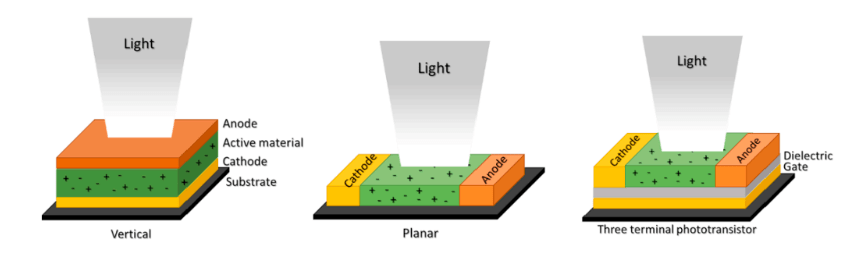
Figure 3. An example of one of the most commonly printed photodetector structures.
Biological Imaging
In biomedical applications, lead sulfide quantum dots serve as fluorescent probes for imaging and diagnostics. Their emission in the NIR region provides deep tissue penetration and minimal autofluorescence from biological tissues. This allows for clearer and more precise imaging in medical diagnostics, enabling better visualization of cellular and molecular processes.
Light-Emitting Diodes (LEDs)
PbS QDs are employed in the production of LEDs due to their tunable emission properties. By adjusting the size of the QDs, the emission wavelength can be precisely controlled, allowing for the creation of LEDs with specific colors. PbS QD LEDs have potential applications in display technologies and lighting.
Sensors
PbS QDs are used in the development of various sensors, including gas sensors and chemical sensors. Their high surface area and reactive surface make them sensitive to changes in the environment, allowing for the detection of gases and other chemical substances at low concentrations. This makes them useful in environmental monitoring and industrial applications.
Can doping with nanoparticles be more beneficial in sensors? Learn now.
Thermoelectric Devices
PbS QDs are explored for their potential in thermoelectric devices, which convert heat into electrical energy. The quantum confinement effects in PbS QDs can enhance their thermoelectric properties, making them promising materials for waste heat recovery and other energy conversion applications.
Memory Devices
Research is ongoing into the use of PbS QDs in memory devices. Their unique electronic properties may allow for the development of new types of non-volatile memory with improved performance and stability compared to traditional materials.
Conclusion
Lead sulfide (PbS) quantum dots (QDs) stand out as a pivotal advancement in nanotechnology, offering unique optical and electronic properties that drive innovation across numerous fields. From their tunable size-dependent bandgaps to their impressive stability and infrared interaction, PbS QDs are integral to the development of cutting-edge applications in photovoltaics, biological imaging, optoelectronics, and more. The diverse synthesis methods—chemical, physical, and biological—further enhance their versatility and potential for large-scale integration into various technologies.
To follow the latest developments and innovations related to nanotechnology, visit Blografi.
References
Can Doping with Nanoparticles be More Beneficial in Sensors? - Nanografi Nano Technology. (n.d.). Retrieved June 4, 2024, from https://nanografi.com/blog/can-doping-with-nanoparticles-be-more-beneficial-in-sensors/
Hou, B., Cho, Y., Kim, B. S., Ahn, D., Lee, S., Park, J. B., Lee, Y. W., Hong, J., Im, H., Morris, S. M., Sohn, J. I., Cha, S. N., & Kim, J. M. (2017). Red green blue emissive lead sulfide quantum dots: heterogeneous synthesis and applications. Journal of Materials Chemistry C, 5(15), 3692–3698. https://doi.org/10.1039/C7TC00576H
Light Spectrum | Experiment on Light Spectrum in the Classroom. (n.d.). Retrieved June 4, 2024, from https://www.vedantu.com/evs/light-spectrum
Moreels, I., Lambert, K., Smeets, D., De Muynck, D., Nollet, T., Martins, J. C., ... & Hens, Z. (2009). Size-tunable, bright, and stable PbS quantum dots: A surface chemistry study. ACS Nano, 3(10), 3023-3030. https://doi.org/10.1021/nn900863a
Oliveira, J., Brito-Pereira, R., Gonçalves, B. F., Etxebarria, I., & Lanceros-Mendez, S. (2019). Recent developments on printed photodetectors for large area and flexible applications. Organic Electronics, 66, 216–226. https://doi.org/10.1016/J.ORGEL.2018.12.028
Synthesis, Properties and Applications of Quantum Dots - Nanografi Nano Technology. (n.d.). Retrieved June 4, 2024, from https://nanografi.com/blog/synthesis-properties-and-applications-of-quantum-dots/
Yun, L., Zhao, W., Avgouropoulos, G., & Lifshitz, E. (2021). PbS Quantum Dots Saturable Absorber for Dual-Wavelength Solitons Generation. Nanomaterials 2021, Vol. 11, Page 2561, 11(10), 2561. https://doi.org/10.3390/NANO11102561
Recent Posts
-
Turning Noise into Power: Energy Harvesting with Piezoelectric Nanogenerators
Ambient acoustic energy, once an untapped resource, is now being converted into sustainable electric …5th Mar 2025 -
Holey Super Graphene in Li-ion Batteries: Next Generation of Energy Storage
Holey Super Graphene (hG), also referred to as “holey graphene,” is redefining li-ion ba …7th Feb 2025 -
Future Communication with 5G Technology and Advanced Materials
5G technology opens the doors to a new era in communication with faster connection speeds, low laten …6th Feb 2025






