Molybdenum Oxide Nanoparticles - Nanografi Blog
Nowadays, oxidation reactions play a substantial role in modern chemistry as well as chemical industry. Around 30% of total production in the chemical industry is carried out by the oxidation process. It means that after polymerization, oxidation is the largest process in the production of chemicals.
In the last few decades, transition metal oxides have attracted the research community in great deal due to their diversified applications. Among them, molybdenum oxide has been found to be one of the most fascinating materials due to its unique structural, optical, electrical, and mechanical properties and multidirectional applications such as gas sensing, photocatalysis, field emission (FE), capacitors, resistive switching, solar cells, light emitting diodes, hole transport materials, etc. Molybdenum is a Block D, Period 5 element, while oxygen is a Block P, Period 2 element in the periodic table. Molybdenum does not occur naturally as a free metal on EarthMolybdenum oxide is a wide bandgap material whose bandgap varies from insulating MoO3 (≈3.2 eV) to more conducting MoO3-x to semi-metallic MoO2 (≈2.55 eV). Molybdenum oxide exists in several phases such as MoO3 (orthorhombic, monoclinic, and hexagonal), Mo4O11 (monoclinic and orthorhombic), Mo5O14 (tetragonal), Mo8O23 (monoclinic), Mo9O26 (monoclinic), and MoO2 (monoclinic) depending upon the oxygen concentration and the oxidation state (+6, +5, +4, and +2) of Mo. The commonly used molybdenum oxide, MoO3, have three different polymorphs and these are:
i) a thermodynamically most stable orthorhombic structure(α-MoO3)
ii) a metastable monoclinic structure (β-MoO3) and
iii) a metastable hexagonal structure (h-MoO3).
Molybdenum oxide nanoparticle, also known as molybdenum trioxide nanoparticle, is a fine light blue powder composed primarily of MoO3 particles with diameters of 100nm or less generally.
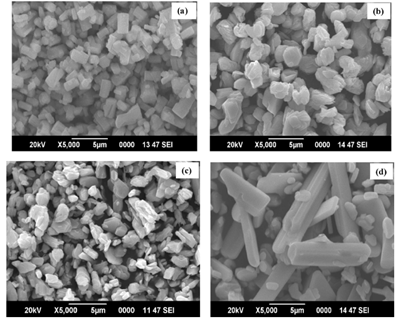
Figure 1: SEM image of MoO3 nanoparticles at different annealing temperatures (a) as-prepared, (b) 400 °C, (c) 500 °C and (d) 600 °C [1].
Due to high melting point, high chemical and thermal stability, and metallic conductivity, monoclinic MoO2 has attracted much attention in the research community.Looking to its high chemical stability, quick oxidation-reduction process, easy nanometric sized particles preparation procedure, we selected molybdenum oxides as viable candidates. Mo exists in three oxidation states and thus can readily participate in redox reactions and exhibits interesting metallic electrical conductivity. Hence, the nanostructured molybdenum oxide particles (MoOx NPs) could act as efficient electron redox mediators, contributing to the electrochemical sensing of some biological compounds such as dopamine. MoOx NPs are widely used in lithium ion batteries, capacitors and gas sensors, while their application in bio-sensing is still limited. Different types of MoO2 morphologies such as nanoparticles, hollow spheres, sheets, large scale nanowires, and nano stars have been synthesized via diversified techniques such as hydrothermal reaction, thermal evaporation, solution-based reactions, and hydrogenation processes. Molybdenum dioxide is also an unusual transition metal oxide because of its high metallic-like electrical conductivity, which is associated with its mixed interatomic bonding and a relatively high density of states at the Fermi level. The existence of free electrons in this region enhances the catalytic activity of Mo4+ in MoO2, unlike that of Mo6+ in MoO3, where all the valence electrons of the metal are covalently bonded to neighboring oxygen atoms. The metallic conductivity of MoO2 makes it a material of interest for many applications, including catalysis. Most recently it has been studied as a possible anode for lithium-ion batteries.
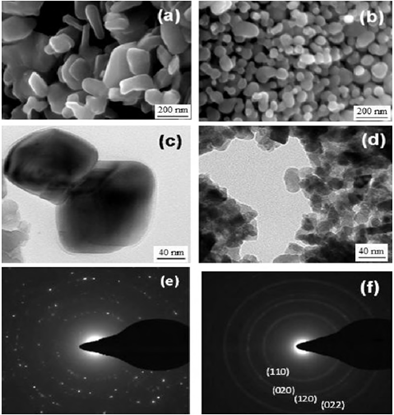
Figure 2: SEM and TEM images of commercially available MoO2 (a and c) and nanoparticle MoO2 (b and d). The corresponding selected area electron diffraction patterns asshown in (e) and (f), respectively. The index of electron diffraction pattern for nanoparticle MoO2 (f) matches to that of pure bulk MoO2[2].
Different morphologies of MoO3 nanostructures (NSs) such as nanoribbons, nanobelts, nanorods, nanowires, nanotubes, and nanosheets are prepared by various physical and chemical methods. Different techniques are deployed to synthesize MoO3 NSs successfully such as chemical vapor deposition (CVD), thermal evaporation, e-beam evaporation, pulsed laser deposition, sputtering, hydrothermal, molecular beam epitaxy (MBE), and van der Waals epitaxy.
MoO3 is a promising candidate to realize applications in electronic, photo-catalytic, gas sensor, biological activity, electrochromic, photochromic, lithium-ion batteries etc., owing to its tunable properties. It is an n-type wide bandgap semiconductor with three crystal structures (i) orthorhombic (α-MoO3) which is thermodynamically stable, (ii) metastable hexagonal (h-MoO3) and (iii) monoclinic (m-MoO3). Orthorhombic (α-MoO3) phase is widely studied by researchers because it gives out many applications. Molybdenum oxide nanoparticles can be synthesized through various methods like hydrothermal, microwave irradiation, co-precipitation, sono-chemical, flame synthesis, sol-gel etc. Among these methods, co-precipitation is a simple and cost-effective method capable producing large quantity of yield. There are many influencing synthesis parameters like acid or base, PH, surfactant, source material, annealing temperature etc., which could change the property of the bulk material significantly.
Laser Ablation of Solids in Liquids
The so-called LASL technique is an effective method to obtain nanostructures. This method is promising since the NPs formed can be free of both surfactants and other ions that exist during chemical synthesis, and it differs from laser ablation in vacuum or gaseous environments since the liquid can help to control some of the parameters of fabrication and to obtain the desired morphology and microstructure. In the LASL process, material is removed from the surface of a target in the form of plasma by the application of a high pulsed laser beam. Usually a target is submerged in a liquid and the laser is focused on the target through the transparent medium. When using the LASL method with metals, there are two main formation mechanisms proposed for the generation of nanostructures:
i) The thermal evaporation with liquid interaction, and
ii) The explosive ejection of nanodroplets. In the former, the formation of nanostructures is associated with the combination of ultrafast quenching of hot plasma and its interaction with surrounding media
In the latter case, it is suggested that the laser irradiation could cause a local melting from the metal target, the adjacent liquid layer is heated to vapor or plasma state with a high pressure, which splashes the molten target into nanodroplets that react with the liquid medium and create the final nanostructures.
Click Image to Learn Also About Molybdenum Sputtering Targets
Reduction of Molybdenum Trioxide Powder
Nanoparticle MoO2 is synthesized by reduction of molybdenum trioxide (MoO3) powder in a 1:3 volume ratio of ethylene glycol to distilled water. The mixture is combined in a Teflon-lined general-purpose vessel, which is subsequently sealed and heated to 180℃ for 12 hours. The liquid ratio of 1:3 was chosen because it yields pure single phase MoO2, without the need for any post-synthesis reduction. After cooling, the dark colored MoO2 was filtered and air dried at 100℃. It is relevant to note that the process used to produce the MoO2 nanoparticles is scalable and large quantities of catalyst could easily be prepared.
Applications
For molybdenum oxide nanoparticles, there are various types of applications. The key applications of molybdenum oxide nanoparticles are as follows:
- In electrochemical capacitors
- In coatings, nanowires, nanofibers, plastics, and textiles
- In specific alloy and catalyst applications
- As catalysts, oxidation catalysts, cracking catalysts, hydrogenation catalysts, and pigments
- In ceramics and glass production
- As a raw material for the production of molybdenum metal.
Catalyst applications: Like many nanomaterials, molybdenum oxide has several applications as a catalyst and is the subject of substantial research. Current known uses include hydrogenation catalysis and cracking catalysis.
Electrochemical applications: The unique electrical and chemical traits of molybdenum oxide at nanometer scales makes it of particular interest in the production of various displays and electrochemical devices.
Material production applications; Probably the primary current usage of molybdenum oxide lay in its versatility in the production of different materials. It serves as a seed material for producing various related substances, such as molybdenum metal, and is used as an additive and ingredient in various glasses, ceramics, and other compound materials.
Pigment applications: The unique optical properties of molybdenum oxide make it of occasional use as an additive for pigmentation.
REFERENCES
1.G. Pradeesh, V. Ponnuswamy, B. Gowtham, R. Suresh, J. Chandrasekaran, Influence of annealing temperature on the properties of molybdenum oxide nanoparticles prepared through chemicalprecipitation method for p-n junction diode application, Optics, 6 September 2018.
2.S. Muthamizh, R. Suresh, K. Giribabu, R. Manigandan, S. Praveen Kumar, S. Munusamy, A. Stephen, and V.Narayanan, Molybdenum oxide Nanotubes: Synthesis and characterizations, https://doi.org/10.1063/1.4917796 Published Online: 25 June 2015
3.J. Appl. Phys. 127, 025301 (2020); https://doi.org/10.1063/1.5127227 Submitted: 12 September 2019. Accepted: 21 December 2019. Published Online: 08 January 2020
4.G. Pradeesh, V. Ponnuswamy⁎, B. Gowtham, R. Suresh, J. Chandrasekaran, Influence of annealing temperature on the properties ofmolybdenum oxide nanoparticles prepared through chemical precipitation method for p-n junction diode application, Optics, 6 September 2018.
5.Le Xin Song, Mang Wang, Shu Zhen Pan, Jun Yang, Jie Chen and Jing Yang, Molybdenum oxide nanoparticles: preparation, characterization, and application in heterogeneous catalysis, Journal of Materials Chemistry, 24 March 2011.ssss
REFERENCES FOR IMAGES
1.G. Pradeesh, V. Ponnuswamy, B. Gowtham, R. Suresh, J. Chandrasekaran, Influence of annealing temperature on the properties of molybdenum oxide nanoparticles prepared through chemicalprecipitation method for p-n junction diode application, Optics, 6 September 2018.
2.Oscar Marin-Floresa, Timothy Turbab, Caleb Ellefsonb, Kang Wangb, Joe Breit c, Jeongmin Ahnb, M. Grant Nortonb, Su Haa, Nanoparticle molybdenum dioxide: A highly active catalyst for partial oxidation of aviation fuels, 2 June 2010.
Recent Posts
-
Turning Noise into Power: Energy Harvesting with Piezoelectric Nanogenerators
Ambient acoustic energy, once an untapped resource, is now being converted into sustainable electric …5th Mar 2025 -
Holey Super Graphene in Li-ion Batteries: Next Generation of Energy Storage
Holey Super Graphene (hG), also referred to as “holey graphene,” is redefining li-ion ba …7th Feb 2025 -
Future Communication with 5G Technology and Advanced Materials
5G technology opens the doors to a new era in communication with faster connection speeds, low laten …6th Feb 2025







