Zinc Carbonate
Zinc carbonate is a chemical compound also known as zinc spar, smithsonite or turkey fat. It is an ore of zinc with a chemical formula ZnCO3. It belongs to organic carbonic acids and derivatives family.
Zinc carbonate is a white and odorless crystalline solid, sub-micron or nano-powder which is insoluble in water, alcohol or acetone. Zinc carbonate also exhibits fluorescence when exposed to ultraviolet light, making it an interesting subject for mineral collectors and researchers in material science. Additionally, it plays a crucial role in various industrial applications, such as rubber manufacturing and paint production, where it serves as a valuable source of zinc and as a pigment. Explore the cutting-edge applications of zinc carbonate by visiting Nanografi's website, a leading provider of advanced nano-materials for research and industry.
Introduction
Zinc Carbonate (ZnCO3) is soluble in alkalis and acids. It is generally transparent and colorless in pure form, but it is colored by the presence of iron, manganese, copper or other elements when it is not in the pure form. Zinc carbonate is an important zinc source which can be easily converted to other zinc compounds such as zinc oxide. This process can be achieved by heating which results in the formation of zinc oxide and carbon dioxide. This process is also known as calcination. Also, this reaction can be achieved by reacting zinc carbonate with dilute acids. It is noteworthy that zinc carbonate as an inorganic salt is commonly used as a catalyst in organic synthesis reactions. Furthermore, this inorganic compound is an appropriate precursor for the fabrication of zinc oxide particles.
Features of Zinc Carbonate
The chemical compound with the formula ZnCO3, known as zinc carbonate, is characterized by its white color. It exhibits an oxidation number of +2, indicative of zinc's valence state within the compound. The density of zinc carbonate is measured at 3.5 g/cm^3, and it has a molecular weight of 125.4 g/mol. It transitions from solid to liquid at a melting point of 333.6 °C when subjected to atmospheric pressure (1 atm). Upon further heating, zinc carbonate reaches its boiling point at a significantly higher temperature of 1970°C. These physical and chemical properties define zinc carbonate's behavior and utility in various applications.
Synthesis of Zinc Carbonate
There are many different methods for synthesizing zinc carbonate. Particles having size range of 10 to 100 nm can be produced with different techniques. Each method has its own advantages and disadvantages. Synthesizing nano particles plays an important role on the performance of the zinc carbonate because nano particles have higher specific surface area which enhances the performance of the powder in industrial uses. For example, it is used to remove toxic gases in respirators. In this application, surface area is a crucial property since it directly affects the interaction area between the toxic gases and zinc carbonate. Also, it has high dispersibility which minimizes reaction time. Some of the synthesis routes for zinc carbonate are given below.
Hydrothermal Method
Hydrothermal synthesis of zinc carbonate as a result of the reaction of ZnCl2 and K2CO3.
Micro-Emulsion Method
Zinc carbonate nanoparticles can be produced via micro-emulsion method in which two reactants that are dissolved in the aqueous nanodroplets of two separate micro emulsions are mixed.
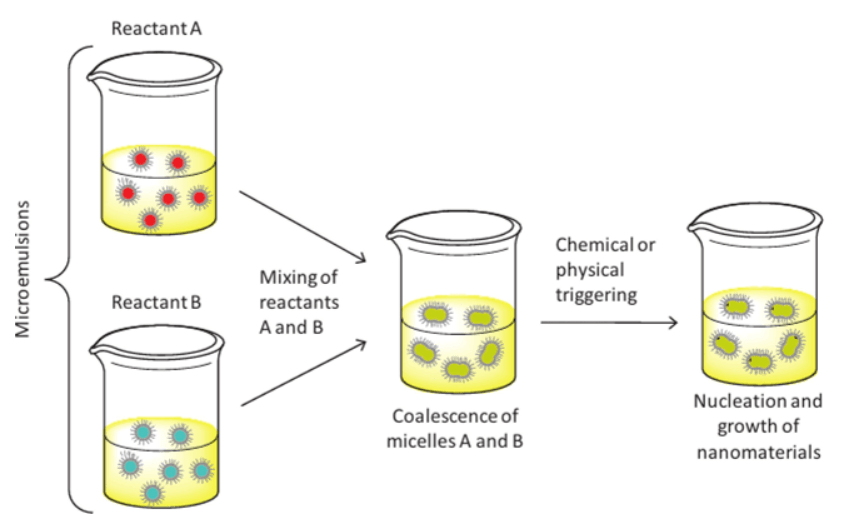
Figure 1. Structure of Microemulsion Method.
Solid-Solid Reaction
Nanocrystalline zinc carbonate can be synthesized via solid-solid reaction by grinding ZnSO4 7H2O and NH4HCO3 with surfactant OP thoroughly at room temperature.
Direct Precipitation
A water soluble zinc salt which is generally zinc sulfate is reacted with sodium carbonate or sodium bicarbonate. Zinc carbonate nano powders are precipitated as a result of this reaction. The product is washed and dried. This powder should be kept free of acidic vapors since it reacts with acids.
ZnSO4(aq) + Na2CO3(aq) → Na2SO4(aq) + ZnCO3(s)
In addition to these, zinc carbonate can be used to produce zinc salts by reacting with acids. Some of the examples are given below.
Hydrochloric Acid + Zinc Carbonate → Zinc Chloride + Carbon Dioxide + Water
- 2HCl + ZnCO3 → ZnCl2 + CO2 + H2O
Sulphuric Acid + Zinc Carbonate → Zinc Sulphate + Carbon Dioxide + Water
- H2SO4 + ZnCO3 → ZnSO4 + CO2 + H2O
Nitric Acid + Zinc Carbonate → Zinc Nitrate + Carbon Dioxide + Water
- 2HNO3 + ZnCO3 → Zn(NO3)2 + CO2 + H2O
To get more information about properties and applications of zinc carbonate, you can read our blog.
Application Areas of Zinc Carbonate
The use of zinc carbonate in industry has been increasing continuously. It can be used in many areas such as cosmetics to agriculture or rubber production to galvanizing process. Also, zinc carbonate is used as precursor to synthesize other types of zinc compounds such as oxides or salts. These compounds have also large application area which indirectly increases the use of zinc carbonate in industry. These industries keep growing and market demand for the zinc carbonate is increasing.
Rubber Production
Zinc carbonate is extensively used in rubber production as a raw material. It is added to improve the translucency or transparency of the natural rubber material because these two materials have very similar refractive index. However, it can also be used with more brightly pigmented rubber as it acts as a curing agent which speed up the hardening of the rubber’s surface. It can also be used as fire retarding material by acting as a fire-proofing filler for the rubbers which are exposed to flame temperature.
Rubber products which use zinc carbonate: Auto parts, shoe sole, rubber bands, gloves, foam compounds, car tires, sporting goods.
Cosmetics
Cosmetic industry is one of the most promising market for the use of zinc carbonate due to its fungicide and antiseptic properties. It is used in wide range of products such as bath, make up, personal cleanliness, shaving, oral care and skin & hair care products. Since it enhances the transparency, it is extensively used to create sunscreen creams. On the other hand, it can also be used as an opacifier in shampoo to give liquid a cloudy appearance.
Titanium Dioxide (TiO2) also has an important use in cosmetics. To learn more, read our blog post.
Animal Feed
Zinc carbonate is used as an animal feed additive. Lack of zinc carbonate can restrict the animal’s growth since it has an important contribution to bone development. Zinc is a vital element within insulin since it provides enzymes with its structure and allows them to function. Pigs and poultry are especially at risk and will generally show poor appetite.
Sputtering Target
Zinc carbonate is also used in thin film applications. It can be used as sputtering target in physical vapor deposition methods. It provides high film density and small grain size for use in semiconductors.
Galvanizing
Galvanizing is an important process to protect steels from corrosion. Freshly galvanized steel reacts with the surrounding and form zinc corrosion products. In air, newly exposed zinc reacts with oxygen to form very thin zinc oxide layer. This zinc oxide layer forms zinc hydroxide in the presence of moisture. This zinc hydroxide reacts with carbon dioxide within the air. The final corrosion product is zinc carbonate which is stable, thin and tenacious film. This process decreases the corrosion rate dramatically.
Medicine
Zinc carbonate is a crucial material in medicine. It is used in respiratory systems to remove toxic gases such as SO2 and HCN. In this application powder size directly related to performance. More surface area means more interaction with other gases. Thus, nano-sized particles are preferred in this application.
Agriculture
Most of the farmers around the world use chemical fertilizers extensively because of its advantages such as low cost and availability. Zinc is an essential micronutrient for the growth and development of plants. It is required in small amounts, but it runs very critical functions such as maintenance of structural integrity of biological membranes, protein synthesis and gene expression.
What is the role of nanotechnology in crop protection in this context? Learn now.
In addition to these areas, zinc carbonate is also used in petroleum industry as Sulphur absorber. It also has antiseptic properties which makes it possible to use in pharmaceutical industry. Also, zinc carbonate has strong white pigmentation. It is used in paints and ceramics due to this property.
Health and Environmental Issues
Zinc carbonate is hazardous to the human health and environment. Zinc intoxications can occur from inhaling zinc particles and oral ingestion results in an excess of zinc in dietary supplements. Stomach acid dissolves metallic zinc to give corrosive ZnCl2 which gives damage to the stomach. These can cause coughing, wheezing, stomach pain etc. Also, zinc carbonate can cause fire or explosions if it is exposed to source of ignition such as open flame. Basically, hazardous nature of zinc carbonate is the drawback of its usage for the green designs.
Conclusion
All in all, zinc carbonate, which is an ore of zinc, is an essential material for many applications. Demand for the zinc carbonate has been increasing since its application areas such as cosmetics, agriculture or rubber production are growing. Depending on the application area, production scale or structure there are different synthesis routes for zinc carbonate. Zinc nano-powder is extensively used in mentioned application areas such as rubber production, respiratory systems or cosmetics. Size of the powder affects performance of the material. Lastly, use of zinc carbonate seems to increase in the future due to growing cosmetic, rubber and agriculture industries.
To get more information, you can visit Blografi.
References
Dobrydnev, S. V., Molodtsova, M. Y., & Kizim, N. F. (2014). Synthesis and study of basic zinc carbonate. Russian Journal of Inorganic Chemistry, 59(8), 798–800. doi:10.1134/s0036023614080038
Hydrothermal synthesis - Wikipedia. (n.d.). Retrieved February 5, 2024, from https://en.wikipedia.org/wiki/Hydrothermal_synthesis
Microemulsion, Template-Assisted Synthesis. (n.d.). Retrieved February 5, 2024, from https://ebrary.net/191958/engineering/microemulsion
Nanotechnology for Crop Protection - Nanografi Nano Technology. (n.d.). Retrieved February 5, 2024, from https://nanografi.com/blog/nanotechnology-for-crop-protection/
Pourmortazavi, S. M., Marashianpour, Z., Karimi, M. S., & Mohammad-Zadeh, M. (2015). Electrochemical synthesis and characterization of zinc carbonate and zinc oxide nanoparticles. Journal of Molecular Structure, 1099, 232–238. doi:10.1016/j.molstruc.2015.06.044
Reilly, C. (2008). The nutritional trace metals. S.l.: Wiley InterScience.
Shamsipur, M., Pourmortazavi, S. M., Hajimirsadeghi, S. S., Zahedi, M. M., & Rahimi-Nasrabadi, M. (2013). Facile synthesis of zinc carbonate and zinc oxide nanoparticles via direct carbonation and thermal decomposition. Ceramics International, 39(1), 819–827. doi:10.1016/j.ceramint.2012.07.003
Smithsonite - Wikipedia. (n.d.). Retrieved July 15, 2020, from https://en.wikipedia.org/wiki/Smithsonite
The properties and application areas of Zinc Carbonate (ZnCO3) nanoparticles - Nanografi Nano Technology. (n.d.). Retrieved February 5, 2024, from https://nanografi.com/blog/the-properties-and-application-areas-of-zinc-carbonate-znco3-nanoparticles/
Titanium Dioxide Reshaping the Field of the Cosmetic Industry - Nanografi - Nanografi Nano Technology. (n.d.). Retrieved February 5, 2024, from https://nanografi.com/blog/titanium-dioxide-reshaping-the-field-of-the-cosmetic-industry-nanografi-/
Xu, B., Peng, B., Cai, B., Wang, S., Wang, X., & Lv, X. (2016). Facile and Selective Synthesis of Imidazobenzimidazolesviaa Copper-Catalysed Domino Addition/Cycloisomerisation/ Coupling Process. Advanced Synthesis & Catalysis, 358(4), 653–660. doi:10.1002/adsc.201500455
Zinc Carbonate | ZnCO3 | CID 19005 - PubChem. (n.d.). Retrieved July 15, 2020, from https://pubchem.ncbi.nlm.nih.gov/compound/Zinc-carbonate
Zinc Chloride | ZnCl2 | CID 5727 - PubChem. (n.d.). Retrieved February 5, 2024, from https://pubchem.ncbi.nlm.nih.gov/compound/Zinc-Chloride#section=3D-Status
Recent Posts
-
Nanocomposites in Food Packaging
The utilization of nanocomposites in food packaging represents a significant advancement in the fiel …19th Apr 2024 -
What is the Difference Between 7075 and 6061 Aluminum Alloy?
When comparing 7075 aluminum alloy to 6061 aluminum alloy, it's essential to understand their disti …5th Apr 2024 -
Iron-Air Batteries: The Ultimate Guide
Iron-air batteries represent a significant breakthrough in energy storage technology, offering a sus …29th Mar 2024







