Tin (Sn) Powder
Tin (Sn) Powder is a versatile metallic substance derived from the element tin, which is represented by the symbol Sn in the periodic table. Known for its unique properties such as high electrical conductivity and excellent solderability, this powder has become privotal in various industries.
From electronics to metallurgy, tin powder's applications are vast, often used in optoelectronics, device manufacturing and as a catalyst. Its attributes combined with its cost-effectiveness, position it as a material of keen interest in ongoing research and commercial applications. Discover the advantage of Nanografi with our tin powder that boasts superior conductivity, ideal solderability, and the purest quality. Explore with us today!
Introduction
Properties of Tin Powder
The metal powders are finding applications in optoelectronics, different kinds of devices, electronics, specialized alloys, information storage and catalysis. Among various metal powders, tin powder is one of the inspected one because of its catalytic, chemical, electrical and antifungal/antibacterial properties. Tin powders are still being researched a lot due to its high electrical conductivity, low electrochemical migration behavior, excellent solderability and low material cost. Tin is one of the most widely used materials in the world. It has a great significance in all industries, particularly in the metallurgy industry due to its low cost.
Tin is a chemical element with the symbol Sn and atomic number 50. Tin is a post-transition metal in group 14 of the periodic elements. It is obtained chiefly from the mineral cassiterite, which contains stannic oxide, SnO2. Tin is the 49th most abundant element on Earth and has, with 10 stable isotopes, the largest number of stable isotopes in the periodic table, due to its magic number of protons. It has two main allotropes, at room temperature, the stable allotrope is β-tin, but at lower temperatures, it transforms into the less dense grey α-tin, which has the diamond cubic structure. Tin melts at relatively low temperatures of about 232℃, which is the lowest in group 14, and its density is approximately 7.00 g/cm3. Tin is a soft, malleable, ductile, and highly crystalline silvery white metal. It is a corrosion-resistant material.
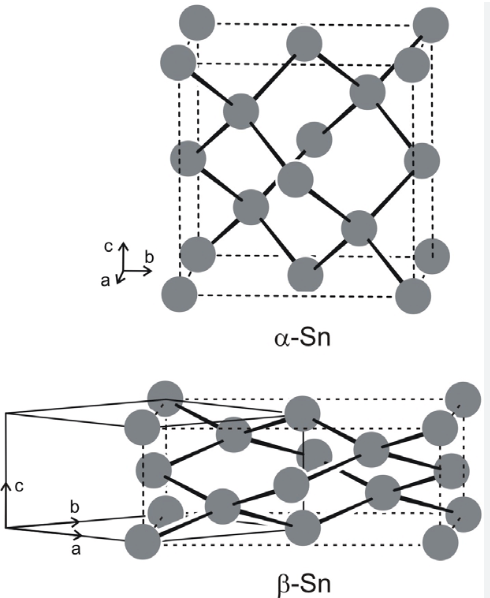
Figure 1. The structures of α-and β-Sn.
Production of Tin Powder
Gas Atomization Process
Gas atomization process is widely used for production of various metal and pre-alloyed powders. And it is also used for production of tin powders. The process involves the disintegration of a liquid stream of molten metal into liquid metal droplets by the impingement of high-pressure gas jets. The liquid metal droplets subsequently cool and solidify into metal powder particles, which can typically range from 1 to 150 µm. In the gas atomization process, high pressure jets of gas (air, argon, nitrogen or helium) is used to disintegrate a stream of molten metal into small droplets which further solidify in fraction of seconds by the quenching effect of gases. Different types of nozzles can be designed to maximize the production rate and to control the characteristics of powders. In order to avoid the oxidation of the powder particles, the atomization should be carried out in a closed chamber under a neutral atmosphere.
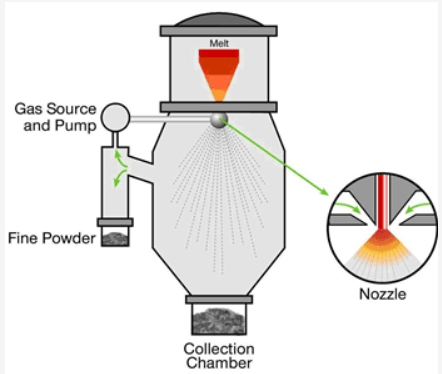
Figure 2. Schematic gas atomization process.
Cementation Process
The cementation (contact exchange) reaction is a special case of electrode-deposition which requires no supply of electrical energy. It has already been demonstrated that, like electrolysis, cementation as a method of producing finely divided copper makes it possible to regulate the properties of powders of importance in powder metallurgy, i.e., obtain powdered copper possessing predetermined properties. The producing tin powder by cementation are still being studied, although tin is one of the heavy nonferrous metals. To get information about nickel oxide powder that can undergo the cementation process and explore its applications, visit our blog.
Chemical Reduction Method
Sn powders can also be synthesized by chemical reduction method. This method is also suitable for the Sn nanoparticle synthesis because the chemical reduction can use a low temperature, resulting in a better control of thermal oxidation of Sn nanoparticles. During the synthesis process, some surfactants are used to protect Sn particles from oxidation.
Applications of Tin Powder
Solder
Tin powder has long been used in alloys with lead as solder, in amounts 5 to 70% w/w. Tin forms a eutectic mixture with lead at the weight proportion of 61.9% tin and 38.1% lead. Such solders are primarily used for joining pipes or electric circuits.
Tin Plating
Tin bonds readily to iron and is used for coating lead, zinc and steel to prevent surface corrosion. Tin-plated steel containers are widely used for food preservation because it is non-toxic, and this forms a part of the market for metallic tin.
Figure 3. Example of tin plating.
Optoelectronics
Tin powders can be oxidized, and the oxides of tin are electrically conductive and transparent. These powders are used to make transparent electrically conducting films with applications in optoelectronic devices such as liquid crystal displays.
Specialized Alloys
Tin forms a variety of alloys with combination of different metals. Tin powder is usually alloyed with copper to make bronze (generally 88% Cu, 12% Sn). Also, tin powders are alloyed with niobium to obtain niobium-tin compound Nb3Sn. The compound is commercially used in coils of superconducting magnets for its high critical temperature (18K) and critical magnetic field (25T). Besides, tin powders are used in zirconium alloys for the cladding of nuclear fuel.
Lithium-ion Batteries
It is known that lithium ion battery performance can be enhanced by incorporating tin powders in the anode electrode, owing to the increase in interfacial area and decrease in lithium ion transport path length.
To explore Nanografi's high-quality lithium battery materials, visit website.
Other Applications
As a pure metal, tin powder is used in the production of electronic valves, and in the production of food packaging for distilled water, beer and carbonated drinks. It can still be used in storage tanks for pharmaceutical chemical solutions, in capacitors electrodes, fuse-wires, ammunitions, tinned iron sheets to protect victuals, sweets or tobacco etc. Besides, the tin powder is used in the production of these papers and of inks and sprays.
Tin powders are primarily used in spray-on applications for corrosion resistance. It is also used in the manufacturing of high-powered magnets and self-lubricating bearings. Did you know that graphene is also used in radiation shielding? To learn more, read our blog.
For industrial applications tin powder with purity of 99.8%, is considered the best grade. The industries dealing in metal replacement, sintered parts, paints, chemicals, metal polishing solder flux, and friction components, use tin powder as their main element. Thus, the excellent result in respective application areas is assured due to the appropriate chemical and physical properties.
Tin powders are used in diamond tooling, powder metallurgy, electronics, radiation shielding diverse applications, markets and technologies by virtue of their unique properties.
Typical applications of tin powder may include pressed and sintered components, bearings and bushings, metallurgical alloying, chemical processes, friction parts, surface engineering, decorative, welding and brazing, etc.
Tin powders are used in manufacturing P/M parts, friction disks, sheathing boards of brakes, clutches, diamond grinding wheels, bronze filters, and also used as the additives of rubbers and plastics, and in the production of chemicals.
In electronic devices, tin and some of its alloys are being used as interconnect materials in on-chip and off-chip applications. Due to their melting point (220–240°C), a high reflow temperature will be needed in the electronics manufacturing process. This high process temperature in electronics assembly has adverse effects not only on energy consumption, but also on substrate war-page, thermal stress and popcorn cracking in molding compounds resulting in poor reliability of the devices. Therefore, studies on lowering processing temperature of Sn are being paid attention.
Conclusion
In a general way, it can be concluded that the formation of Sn powder is relatively sensitive to the synthesis conditions. Therefore, before using a synthesis method described in the literature, one should observe the real influence of synthesis conditions such as temperature, stirring and composition of the mixture for an effective control of size distribution and particle shape. Although Sn particles have some interesting properties, more research should be done in order to enhance the properties and moderate the disadvantages. A lot of researches are still being conducted to improve its properties and lower its manufacturing cost in order to implement its use in more different applications. In the coming years, this material will innovate the world of technology due to its unique characteristics.
An overview of research on tin powders has been presented in terms of structure, properties, synthesis, and application areas. Although tin powders have interesting properties and promising outlook, the research progress on physical aspects and potential applications are limited so far. Therefore, more research and experiments are needed to find and explore new sides of these materials. To support these research endeavors and advance your work with high-quality products, contact with Nanografi.
References
Artamonov, V. P., & Akhmetzhanova, R. R. (1986). Production of tin powder by the cementation process. Soviet Powder Metallurgy and Metal Ceramics, 25(1), 5–7. doi: 10.1007/bf00843005
Dunkly J. J ., “Gas atomization a review of the current state of the art”, Advances in Powder Metallurgy & Particulate Materials, 55-66 (1999).
Gas atomization. Courtesy of LPW Technology, reprinted with permission... | Download Scientific Diagram. (n.d.). Retrieved February 12, 2024, from https://www.researchgate.net/figure/Gas-atomization-Courtesy-of-LPW-Technology-reprinted-with-permission-from-Ref-70_fig2_331798165
Graphene Radiation Shielding Applications - Nanografi Nano Technology. (n.d.). Retrieved February 12, 2024, from https://nanografi.com/blog/graphene-radiation-shielding-applications/
Nickel Oxide Powder and Its Applications - Nanografi Nano Technology. (n.d.). Retrieved February 12, 2024, from https://nanografi.com/blog/nickel-oxide-powder-and-its-applications/
Production of tin powders by ultrasonic atomization and solid state transformation. (1993). Metal Powder Report, 48(3), 50. doi: 10.1016/0026-0657(93)90365-y.
RANJAN PANDA Synopsys, P., Dutt, N. D., & Nicolau, A. (1084). On-Chip vs. Off-Chip Memory: The Data Partitioning Problem in Embedded Processor-Based Systems.
The structures of α-and β-Sn. The tetragonal unit cell of β-Sn is drawn... | Download Scientific Diagram. (n.d.). Retrieved February 12, 2024, from https://www.researchgate.net/figure/The-structures-of-a-and-b-Sn-The-tetragonal-unit-cell-of-b-Sn-is-drawn-with-a-solid_fig1_250467447
Ullrich W. J., “Production of Tin Powders”, in ASM Handbook, ASM International Publisher, Vol. 7, 349 -350 (1998).
Recent Posts
-
What is the Difference Between 7075 and 6061 Aluminum Alloy?
When comparing 7075 aluminum alloy to 6061 aluminum alloy, it's essential to understand their disti …5th Apr 2024 -
Iron-Air Batteries: The Ultimate Guide
Iron-air batteries represent a significant breakthrough in energy storage technology, offering a sus …29th Mar 2024 -
Discovering the Power of 2D Materials
In material science, the discovery of two-dimensional (2D) materials represents a transformative de …22nd Mar 2024