Paramagnetic Nanoparticles - Nanografi Blog
So far, a lot of applications have been introduced for paramagnetic nanoparticles including in cancer therapy and diagnostics. The progress in nanotechnology and efficient synthesis methods have enhanced the performance of paramagnetic materials and agents.
As we delve deeper into the world of nanotechnology, the remarkable progress in efficient synthesis methods and the enhanced performance of paramagnetic materials are paving the way for groundbreaking applications. To explore the cutting-edge possibilities of nanomaterials, visit Nanografi's website for a glimpse into the future.
Introduction
Important factors in characterizing and designing nanoparticles such as morphology, size surface area and surface chemistry determine their behavior, properties in vivo and in vitro, therapeutic efficacy, the accuracy of diagnostic of magnetic resonance imaging as well as the synthesis and production plan of a variety of paramagnetic nanoparticles. Paramagnetic nanoparticles mainly comprise rare earth metal oxides and hydroxides and that they can serve as potential alternatives to replace iron oxide nanoparticles.
What is Paramagnetism?
Prior to the definition of paramagnetism, it is useful to review what magnetism is. Magnetism basically originates from the spin magnetic moments of elementary particles and electric current. Such a property of materials is because of the magnetic moments of their electrons orbiting atoms. Electrons magnetic moments are thousands of times as much as atoms nuclei magnetic moments and therefore, the portion of nuclei in magnetism is almost negligible. However, it should be noted that nuclear magnetic moments are of a great degree of importance, especially in nuclear magnetic resonance (NMR) and magnetic resonance imaging (MRI).
Could Magnetic Graphene be possible? Learn now!
As a type of magnetic property of matter, paramagnetism is a phenomenon in which one or more unpaired electrons on atoms are attracted to an externally applied magnetic field. During applying a magnetic field, magnetic moments of atoms are excited with the consecutive reversion of them back to the ground state called relaxation upon the removal of the applied field. The concept of magnetic relaxation is described through the parameters denoted as T1 and T2 where T1 is explained as the relaxation state that results when the longitudinal magnetization returns to the equilibrium state while T2 is the return of transverse magnetization to the state of equilibrium. In the case of a constant strength of magnetic field, the T1 and T2 relaxations will differ depending on types of different tissues with diseased tissues possessing distinct values of T1 and T2 compared to healthy tissues 1. Although it is somehow known that their relaxation is partly dependent on their paramagnetism, the properties of paramagnetic particles relaxation have not been explained well. Technically, 1H and 2H NMR relaxations outcomes materials and methods as well as the paramagnetic resonance of electron are employed in order to study the relaxation of paramagnetic particles.
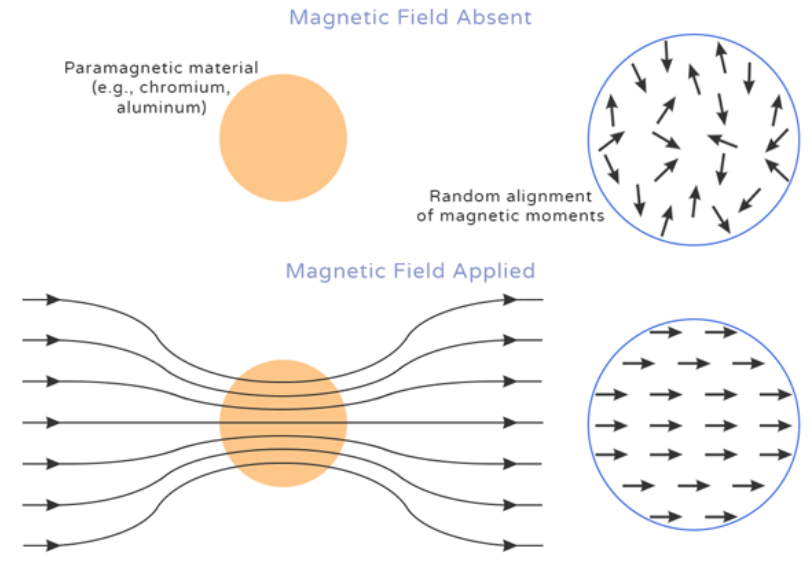
Figure 1. Definition of paramagnetism.
Classification of Paramagnetic Nanoparticles
Lanthanides from the periodic table of elements such as gadolinium are among the most common and favorable paramagnetic materials because of possessing a high number of unpaired valence electrons. Such an electron configuration makes lanthanides promising and excellent T1 shortening metals. The disadvantage of lanthanides is their high toxicity in their free form. Nevertheless, chelating lanthanide metal ions considerably decreases the toxicity level while supporting the practically high magnetic characteristic with applications as contrast agents in magnetic resonance imaging.
The next class of nanoscale magnetic particles is manganese nanoparticles which have replaced gadolinium nanoparticles. Manganese has emerged as an alternative for gadolinium in treatment of patients with renal disorders as well as liver transplantation 1.
Iron oxide nanoparticles, Fe2O3 and Fe3O4, are regarded as superparamagnetic agents with rapidly growing applications in separation science, tracking and cell labeling for the therapeutic purposes in cancer therapy, tumor ablation by hyperthermia and as diagnostic agents. Iron oxide nanoparticles applications depend on the tailored properties such as magnetism, surface chemistry, shape and size 3. In particular, iron oxide nanoparticles are among the most common T2-shortening contrast agents that generate dark contrast when used at a target site. The T2 relaxation quality of iron oxide nanoparticles depend on the morphology and structure of nanoparticles. Iron oxide nanoworms have appeared to possess elevated magnetic relaxation in magnetic resonance imaging (MRI) when compared to spherical iron oxide nanoparticles which is mainly because of the increased orientation of distinct iron oxide cores with their own magnetic moments. In addition to this, studies have demonstrated that iron oxide nanoparticles clustering increases the T2 relaxation so dramatically that chain-like nanoparticles made of three particles show relaxation nearly three times as high as a particle with a spherical shape.
Designing Paramagnetic Nanoparticles
Up to this point, a variety of methods have been suggested and examined to design and prepare paramagnetic nanoparticles. Generally, iron oxide nanoparticles are prepared by co-precipitating ion metal ions namely ferrous and ferric cations considered as the most efficient and simplest technique to synthesize iron oxide nanoparticles in large industrial quantities. There are several factors affecting the particles characterization like shape and size including the Fe2+:Fe3+ ratio, pH of the reaction media, iron concentration, temperature and the ionic strength. The main downside of co-precipitation is the obtained nanoparticles are not homogenous in size. Therefore, the water-in-oil microemulsion technique, as a widely used alternative, is adopted which is through using nanosized water droplets being dispersed in an oil phase along with surfactant molecules used to stabilize them at water and oil interface. The iron oxide nanoparticles synthesized based on microemulsion method have narrower size distribution compared to those by co-precipitation. Another recent method is high-temperature decomposition is regarded as a useful method to generate magnetic nanoparticles in which iron precursors including Fe(acac)c, Fe(Co)5 and Fe(Cup)3 are decomposed in organic solvents containing surfactant molecules with a resulting iron oxide nanoparticles with excellent monodispersity as well as size distribution and size control.
Uses and Applications of Iron oxide Nanoparticles
There is a broad range of applications for magnetic nanoparticles as in separating and purifying biological molecules, protein detection, DNA delivery and tumor suppression by localize heating called hyperthermia. The cancer therapy application of iron oxide nanoparticles is mainly based on the magneto-spin phenomena in reactions that involve free radicals and semiconductor materials capable of generating oxygen radicals. In general, magnetic nanotherapy is done through external control by electromagnetic field reactive oxygen species (ROS) and reactive nitrogen species (RNS).
To learn the use of Iron Oxide nanoparticles within in the petroleum industry, read our blog.
Conclusion
Paramagnetism is generally a property of atoms with unpaired electrons commonly available among lanthanide metals as well as transition metals such as iron, cobalt and nickel. Gadolinium as a lanthanide metal is a commonly used as a temperature-dependent paramagnetic agents with extensive applications. Iron oxide nanoparticles have emerged as a promising nanoscale paramagnetic agents with mostly biomedical applications and cancer therapy.
Discover Nanografi's Iron Oxide Products now, which contribute to many projects and research with their high quality and high performance.
References
Gadolinium Powder - Nanografi Nano Technology. (n.d.). Retrieved January 16, 2024, from https://nanografi.com/blog/gadolinium-powder/
Heo, D. N. et al. Scale-Up Production of Theranostic Nanoparticles. Cancer Theranostics (Elsevier Inc., 2014). doi:10.1016/B978-0-12-407722-5.00024-4.
Iron Oxide Nanoparticles/Nanopowder and Applications - Nanografi Nano Technology. (n.d.). Retrieved January 16, 2024, from https://nanografi.com/blog/iron-oxide-nanoparticlesnanopowder-and-applications/
Is Magnetic Graphene Possible? - Nanografi Nano Technology. (n.d.). Retrieved January 16, 2024, from https://nanografi.com/blog/is-magnetic-graphene-possible/
Paramagnetism: Definition and Examples. (n.d.). Retrieved January 16, 2024, from https://www.sciencefacts.net/paramagnetism.html
The Use of Iron Oxide Nanoparticles within Separation Process in the Petroleum Industry - Nanografi Nano Technology. (n.d.). Retrieved January 16, 2024, from https://nanografi.com/blog/the-use-of-iron-oxide-nanoparticles-within-separation-process-in-the-petroleum-industry/
Toy, R. & Karathanasis, E. Nanomaterials in Pharmacology. (2016) doi:10.1007/978-1-4939-3121-7.
Vuong, Q. L. et al. Paramagnetic nanoparticles as potential MRI contrast agents: Characterization, NMR relaxation, simulations and theory. Magn. Reson. Mater. Physics, Biol. Med. 25, 467–478 (2012).
Recent Posts
-
Nanocomposites in Food Packaging
The utilization of nanocomposites in food packaging represents a significant advancement in the fiel …19th Apr 2024 -
What is the Difference Between 7075 and 6061 Aluminum Alloy?
When comparing 7075 aluminum alloy to 6061 aluminum alloy, it's essential to understand their disti …5th Apr 2024 -
Iron-Air Batteries: The Ultimate Guide
Iron-air batteries represent a significant breakthrough in energy storage technology, offering a sus …29th Mar 2024






