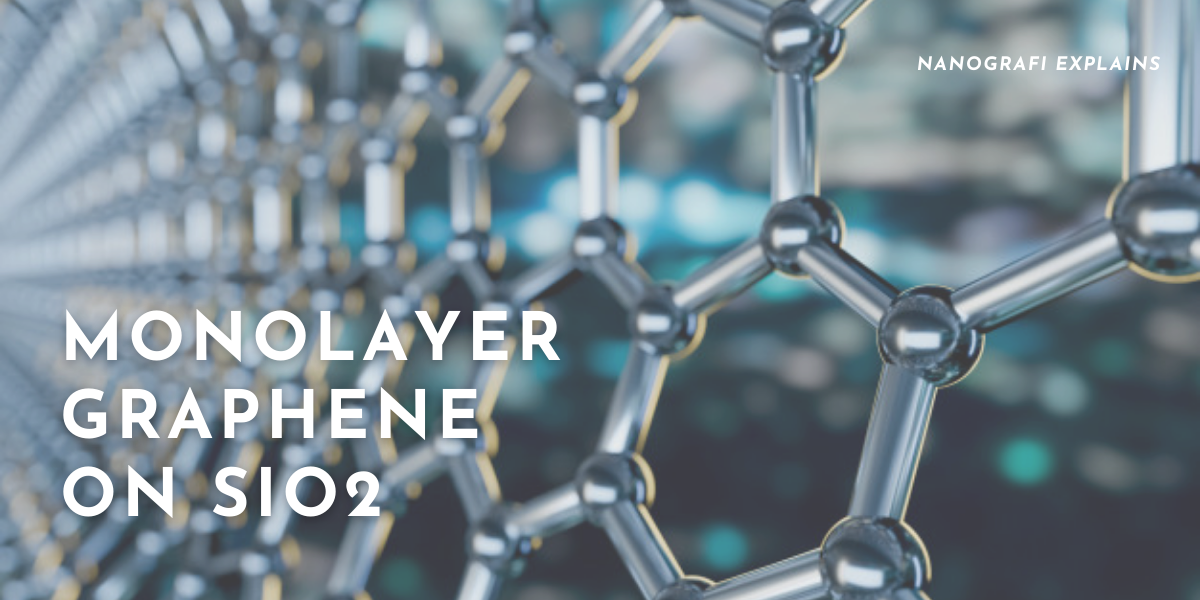
Monolayer Graphene on SiO2/Si Substrate.
Graphene is a world-renowned allotrope of carbon famous for all the remarkable work that it is performing in industries throughout the world.The carbon atoms present in graphene are single-layered and that graphene too has many forms in which it exists.One of its forms is monolayered graphene
In this article, the ways in which monolayer graphene acts with the SiO2 or Si substrate are explained as well as all the remarkable and various properties that are exhibited by graphene as these are actually the building blocks that ultimately enable the graphene to work in all the mysterious yet amazing ways.
Introduction
A carbon’s allotrope, graphene, consists of a single layer of atoms organized in a 2-dimensionalhoneycomb lattice. It is carbon’s graphite allotrope and consists of stacked graphene layers. In a graphene sheet, each atom is connected by σ-bond to its three closest neighbors, and shares one of the electrons to a conduction band, extending over the complete sheet. Same bonding is done in polycyclic aromatic hydrocarbons and carbon nanotubes, and this type of bonding is also partially done in glassy carbon and fullerenes. Graphene is turned into a semimetal with remarkable electronic characteristics because of these conduction bands.
The properties of this semimetal can be explained well by the theoriesfor massless relativistic particles. Graphene’s charge carriers display linear dependence of energy on momentum instead of quadratic. Bipolar conduction is displayed by the field-effect transistors with graphene. Over long distances, the charge transport is ballistic. Nonlinear and large diamagnetism and large quantum oscillations are showed by this material.
Further characteristics
Electricity and heat are conducted very efficiently by graphene along its plane. All of the visible wavelengths of light are absorbed strongly by this material, therefore explaining graphite’s black color. Despite that, due to it being very thin, a single graphene sheet is almost transparent. As compared to any steel of the same thickness, this material is almost about 100 times stronger. For decades, graphene is being theorized by scientists. For centuries, it has been made in little quantities unknowingly by using pencils and graphite's other similar applications. In 1962, it was observed in electron microscopes but it was only studied when it was supported on the surface of the metal.
In 2004, Andre Geim and Konstantin Novoselov rediscovered, isolated, and characterized that material at the University of Manchester, and in 2010, because they researched the material, they were awarded the Nobel Prize in Physics. Graphene of high quality turned out to be very easy to isolate. In 2012, graphene had a global market of $9 million, and most of it was from development and research in composites, electric batteries, electronics, and semiconductors. The name ‘graphite’ was used by the IUPAC (International Union for Pure and Applied Chemistry) for graphene and the 3-dimensional material, only when there is a discussion on the structural relations, reactions, or other characteristics of individual layers. The layer should be isolated from its environment according to the definition of free-standing or isolated graphene, but it also includes the layers that are either suspended or transferred to silicon carbide or silicon dioxide.
Properties of Graphene
Thermal Properties
Due to the potential of graphene for thermal management applications, a huge amount of attention has been gained by thermal transport in graphene as it is an active area of research right now. In comparison to the pyrolytic graphite’s thermal conductivity of 2000 Wm−1K−1 at room temperature, a remarkable large thermal conductivity of 5300 Wm−1K−1 is possessed by the suspended graphene according to the early measurements. Although, such high thermal conductivity measurements couldn’t be reached by the defected graphene, creating a broad range of thermal conductivities between 1500 – 2500 W⋅m−1⋅K−1 for suspended single-layer graphene.
The large measurement uncertainties, variations in the processing conditions, and quality of graphene cause a large range of thermal conductivity. Also, at room temperature, the thermal conductivity is lessened to 500-600 Wm−1K−1 when graphene of single-layer is supported on an amorphous material because of graphene lattice waves’ scattering by the substrate, and for the graphene of few layers, the thermal conductivity can be even lower, for instance in the amorphous oxide. Similarly, for bilayer graphene, the suspended graphene's thermal conductivity can decrease to almost 500-600 Wm−1K−1 due to the contribution of the polymeric residue.
Mechanical
2-dimensional Graphene’s density is 0.763 mg per square meter. The strongest material ever, graphene has 130 GPa (19,000,000 psi) as its intrinsic tensile strength and its stiffness (Young’s modulus) is almost 1 TPa (150,000,000 psi). For stretching large-area freestanding graphene, the tensile strength is ~50-60 GPa. According to the Nobel announcement, a 4kg cat would be supported by a 1 square meter graphene hammock but would weigh only like one of the whiskers of the cat at 0.77 mg which is about 0.001% of the weight of paper’s 1 m2. With negligible strain, a large-angle-bent graphene monolayer has been obtained, displaying a 2-dimensional carbon nanostructure’s mechanical robustness. Monolayer graphene has remarkable carrier mobility even with extreme deformation.
Chemical
The theoretical specific surface area (SSA) of graphene is 2630 m2/g which is much more as compared to the SSA of carbon nanotubes (≈100 to 1000 m2/g) and SSA of carbon black (usually less than 900 m2/g) and is the same as the activated carbon. Only in graphene, every atom is available from both sides for the chemical reaction because of graphene’s 2-dimensional structure. At graphene sheet’s edges, atoms have remarkable chemical reactivity. As compared to any allotrope, graphene possesses the highest ratio of edge atoms.
The chemical reactivity of graphene is increased because of the defects within a sheet. Between the oxygen gas and single-layer graphene's basal plane, the reaction's onset temperature is less than 260 °C (530 K). At extremely low temperatures, graphene starts burning (350 °C (620 K)). X-ray photoelectron spectroscopy and infrared spectroscopy analyze graphene as it is modified commonly with nitrogen- and oxygen-containing functional groups. Although, the structure should be well controlled if there is a need to determine the graphene’s structures with nitrogen- and oxygen-functional groups.
Forms of Graphene
Monolayer sheets
A production unit was presented by a group of Polish scientists in 2013, which enabled the production of continuous monolayer sheets. The growth of graphene on a liquid metal matrix determines the process, the product of which was known asHSMG. Monolayer Graphene is made up of a flat one-atom sp2 carbon atoms’ thick sheet, packed tightly in a honeycomb crystal lattice structure. For fullerenes, carbon nanotubes, and graphite, monolayer graphene is the basic structural element. On Si/SiO2 substrate wafers, the graphene samples are presented as nanoflakes. Each layer has a 0.34 nm thickness, thus being mono-atomically thin, although it is possible for the monolayer graphene to form multi-layered flakes. Flakes can be found by using microscopic imagery and processed easily by using techniques of microelectronic fabrications. One of the best 2-dimensional crystals is graphene as it has excellent mechanical and electronic characteristics.
Graphene is supposed to be a potential breakthrough when it comes to carbon-based nano-electronics due to its structural flexibility, high electronic mobility, and ability to be tuned from p-type to n-type doping by applying the gate voltage. For carbon graphene nanosheets’ applications, research has been focused on platforms like, in the electrodes of high surface area to be used in bio-science, in solar energy cells, in medical imaging devices, in biological sensors, as active materials in field emitter arrays for flat panel screen displays, and next-wave microchips. Wherever carbon nanotubes are being utilized, graphene is a possible potential replacement material for carbon nanotubes.
To get more information about graphene,
you can read our blog post here.
Monolayer Graphene on SiO2/Si Substrate
Over the past, the carbon nanomaterials like graphene and carbon nanotubes have gained a lot of interest and attention because of their remarkable characteristics, and their 2-dimensional and 1-dimensional structures, therefore suggesting possible applications in various fields. There has been an emergence of another interesting carbon nanomaterial recently, known as carbon nanoscroll (CNS). Carbon nanoscroll is a spirally-wrapped 2D graphene sheet with a 1D tubular structure, similar to the structure of a multiwalled carbon nanotube (MWCNT).
High mechanical strength and high carrier mobility are two of the remarkable characteristics of both CNTs and graphene that the carbon nanoscroll inherits. A hybrid structure should also have some different characteristics than the characteristics of carbon nanotubes and graphene. For instance, the π-π interaction between the wrapped graphene’s outer and inner surfaces affects the electronic transport of carbon nanoscroll whereas when it comes to graphene’s flat sheet, there is not much effect on the carbon nanoscroll’s electronic transport. Instead of various coaxially nested graphene cylinders, the flow of electric current is inside a single scrolled graphene layer just like how the electric current flows in the MWCNTs.
Theoretical Calculations
Due to the remarkable topology of CNC, the theoretical calculations assume CNS’s some rare and unique optical and electronic characteristics. Due to the CNS not being a closed topological structure, carbon nanoscroll’s interlayer spaces can be intercalated easily. Relatively, MWCNT’s rigid graphene cylinders will resist lattice expansion because of intercalation. Thus, carbon nanoscrolls can be used in batteries and supercapacitors as they ease hydrogen storage and chemical doping. The diameter of CNS could be utilized in the nanomechanical devices as a nanoactuator because its diameter can be expanded easily by intercalation or charge injection.
Mechanical Exfoliation
Mechanical exfoliation was done of natural graphite to extract graphene for the first time on a degenerately doped Si wafer. SiO2 of 285 nm covers it. The number of their layers was identified according to their Raman spectra after the selection of ideal graphene samples under an optical microscope. Monolayer graphene was usually located for usage. Then the chip was immersed into a Petri dish which was filled for 5 minutes with isopropyl alcohol (IPA), then the chip was picked up and nitrogen was used to dry it. After all of the above-mentioned procedures, it was observed that graphene had surprisingly transformed to a 1-dimensional fiber-like structure from a 2-dimensional sheet.
In order to clear this fiber-like structure, it was transferred to a grid of transmission electron microscope (TEM) for TEM study through the usage of a nanoprobe system. According to the TEM image, it has a structure of a tube with thick walls surrounding the hollow core, and the walls consist of layers of graphene. Other than that, the TEM image also showed that the layers of graphene in the walls are stacked, compactly, and uniformly. 0.35 nm is the distance between the adjacent layers of graphene, just like multi-walled carbon nanotubes and graphite, which means that like them, there is no contamination between these uniformly and compactly stacked graphene layers.
Structure from Graphene Monolayer
According to the TEM results, CNS is the fabricated 1-dimensional structure that is rolled up from a graphene monolayer. The whole process was then recorded and monitored through the usage of a CCD camera on an optical-microscope for clarifying the production of the scroll.
One of the straight edges of monolayer graphene started rolling up when an IPA solution’s droplet (IPA/water ∼ 1:3) was put on the graphene monolayer. Although before the evaporation of the IPA solution, the rolling process stopped, thereby leaving graphene's large part staying flat. Then there was the addition of another pure IPA solution's droplet. The scrolling process didn't stop until the graphene sheet was completely rolled up into a carbon nanoscroll. Later, it was observed that every graphene monolayer could not roll up into CNS. The graphene monolayers which were extremely contaminated or had abnormal shapes stayed unchanged in the IPA solution. It is possible to infer from these observations that the rolling up of the graphene depends on the IPA and both the graphene’s shape and its contamination is sensitive to this process.
Steps of CNS Formation
Below are the following 4 steps that can help in further explaining the process of CNS formation. The qualitative and simple analysis is described below.
1. A surface strain is formed in graphene in the first step because graphene's upper and lower surfaces were in contact with SiO2 and IPA after the chip with graphene was submerged into the IPA solution. In graphene sheet's bending, one of the main factors was this surface strain.
2. The surface strain lifted graphene's edges from the substrate in the second step. Then, graphene’s detachment was facilitated by the entering of IPA molecules into the space between the substrate and graphene.
3. Graphene’s detached parts curved in the third step because of the perturbations in the environment of the solution. Graphene’s total free energy decreases because of the π-π interaction of the overlapped parts when graphene’s free end somehow touched graphene’s another part by chance. However, because of bending, the curvature energy increased.
4. In the final step, the scrolling process kept going on. Graphene detached from the substrate and rolled up little by little until there was a production of carbon nanoscroll with the total free energy facing a decrease.
The Change of Energies
The change in the surface and interfacial energy during the process of scrolling should be calculated for explaining the mechanism quantitatively. The scrolling process is optimized in the following 3 steps and is based on the above analysis. First, IPA of various concentrations was used for adjusting the scrolling speed. It was observed that the pure IPA was too difficult for scrolling, with usually the products having folded graphene instead of carbon nanoscrolls.
There was a preselection of graphene’s shape as the shape of the graphene is a major factor in facilitating scrolling. During the process of scrolling, irregularly shaped graphene sheets were pinned down, leaving an unfinished CNS. However, a long smooth edge was good for scrolling as the sheets were likely to scroll along this edge. Graphene was also protected from contamination as it was observed that graphene which was heavily contaminated, was difficult to scroll. The reason for scrolling being difficult is that graphene's edges and the surface can be modified by contaminations. Thus, if a CNS of high-quality is needed to be produced, then graphene should be protected carefully. Well-stacked CNSs resembling MWCNTs can be obtained after these optimizing procedures.
Resistances Versus Gate
The whole phenomenon of resistances versus gate-voltage data are estimated in the ranges of temperature which have a range from 20 to 300 K, this explains that as soon as the resistance for CNS decreases the temperature on the other hand increases and vice versa. It has been made evident that the gate voltage has a varied range starting from -40 till 40 V, this enables the resistance slope to change in a very smooth manner and as a result, the maximum value at the gate voltage ranges from 0 to 15 V. This is the exact behavior as of the transporting mechanism of a wide graphene nanoribbon. There is a prominent peak known as the Dirac point having the voltage around zero in comparison to the wide and smooth transition. In order to explain the wide transition, a CNS device was noticed as it had a very weak capacitance coupled in comparison to the flat graphene. The result of this is that fewer carriers are obtained under the very exact gate voltage.
To get more information about graphene,
you can read our blog post here.
The Estimated Results
The estimation suggests that this capacitance is about one-twentieth of that between SiO2 and flat graphene, signifying that the measurement probed only the low-carrier-density region around the Dirac point. Due to carriers being activated, CNS’s conductivity increased in this region about 2.2 times from 20 to 300 K. Only in extremely clean 2-dimensional graphene samples, a strong temperature-dependent conductivity like this can be probed, like those suspended ones forged in the vacuum. It is being implied here that the measured CNS device possesses few charge impurities and is clean. This study is the same as the TEM studies that are done above, and therefore there is no additional room left for the impurities that are present in this spacing of 0.35 nm.
The Final Comparison
The final step is the comparison of the characteristics of conduction of current of both the CNS and the MWCNT and in this regard, only the shell that is the outermost one takes a part in the process of conduction at a low bias. The shells that are the inner and innermost ones only couple with the electrodes that are the external ones via a barrier which is basically composed of the outer graphene layer. This visible difference in the process of conduction counts for the low resistance values of the CNS in the diffusive transport region having a low bias.
In order to attain a higher bias transport, linear behavior is shown by the I-V curve as soon as the voltage increases which should be up to ∼2 V, and other than sub-linear behavior is necessary too when higher bias is being exhibited. This entire phenomenon is very different as compared to the I-V curve of the MWCNT exhibited at a high bias as it shows a current saturation in comparison to this. Therefore, it is evident that the CNS is rather of high quality as through CNS a huge current density can be supported. That is why CNSs have a higher possibility of being used for future circuits in order to serve the purpose of microcircuit interconnects.
These results are by far the product of researches that have been done until now but quite a lot more experiments and studies are needed to evaluate this phenomenon to achieve better outcomes and sustain better working mechanics for these products.
Conclusion
Graphene as explained above is an excellent product to work with and so are SiO2 and Si. A research was conducted in this regard and it was made quite evident from that, that the compatibility of monolayer graphene and SiO2 or Si is extremely beneficial and remarkable as it helps carry out a lot of important tasks which pave the way for success for the industries.
To get more information, you can visit Blografi.
References
https://cutt.ly/eJKeDR9
https://www.americanelements.com/monolayer-graphene-1034343-98-0
https://iopscience.iop.org/article/10.1088/2053-1591/aae053/pdf
https://pubs.acs.org/doi/abs/10.1021/acsami.7b01370
Recent Posts
-
What is the Difference Between 7075 and 6061 Aluminum Alloy?
When comparing 7075 aluminum alloy to 6061 aluminum alloy, it's essential to understand their disti …5th Apr 2024 -
Iron-Air Batteries: The Ultimate Guide
Iron-air batteries represent a significant breakthrough in energy storage technology, offering a sus …29th Mar 2024 -
Discovering the Power of 2D Materials
In material science, the discovery of two-dimensional (2D) materials represents a transformative de …22nd Mar 2024