Graphene Oxide Dispersions
Graphene oxide (GO) disperses in several organic solvents such as N-methyl-2-pyrrolidone, N-dimethylformamide, ethylene glycol, and tetrahydrofuran. The graphene oxide solubility has a unique reduction process that is exhibited by dispersion.The graphene oxide dispersion is characterized by more excellent stability for layers ranging between nano-scale to micro-scale.
It is similar to the graphene oxide dispersion in distilled water. However, chlorinated water, a satisfactory level of dispersion was examined after extreme solubilization. This solubilization helps in scaling liquid-phase rGO links for printing flexible electronics.
Introduction
Graphene is a thin layer of graphite-sp2 hybridized carbon atoms-that have carbon atoms arranged like the honeycomb lattice. The structure is only two-dimensional, which enables the carbon allotropes to grow at any dimension. Because Graphene can be small as one single layer, it inhibits several useful applications such as supercapacitors, touch panels, photovoltaics, and biosensors. Good condition graphite films are fabricated using micromechanical exfoliation Chemical vapor deposition (CVD) of Graphene. CVD is a successful process provided that the temperature is kept high, and roll to roll mass production process is compatible.
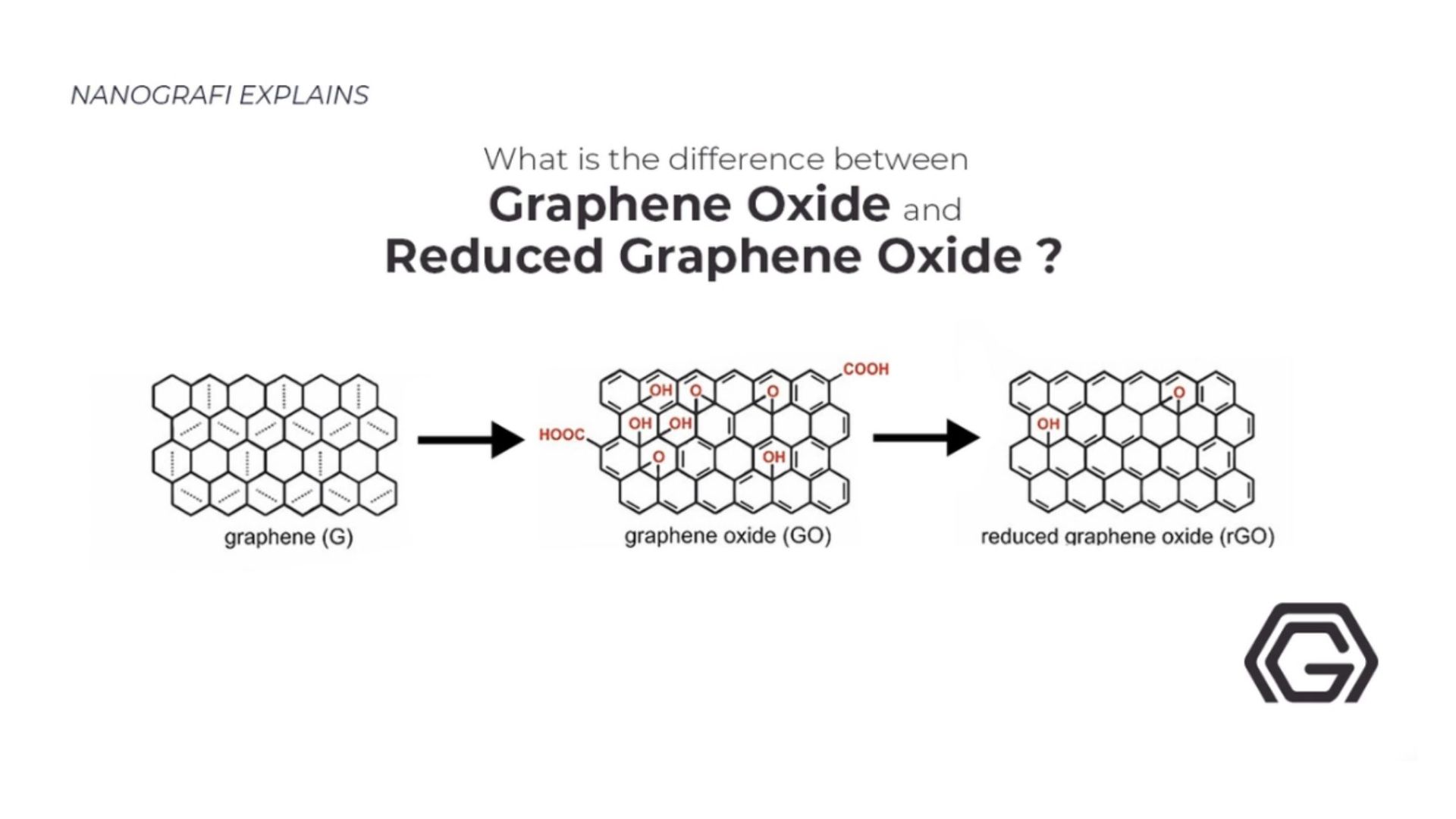
The stabilizer used to exfoliate the Graphene in the liquid medium must be of good quality because the solubility directly depends on the quality of the exfoliator. On the contrary, the solution of processed Graphene from exfoliated graphene oxide (graphene oxide) is a useful process because it can be manufactured in ample quantities in an economical budget. graphene oxide is a special type of oxidized Graphene that has epoxide and hydroxyl groups at basal lanes. Moreover, Go has carboxyl and carbonyl groups at the edges. Graphene can even act as an insulator because the C-O bond can disrupt the sp2 hybridization. Nonetheless, graphene oxide can also be made conductive by removing the oxygen functional groups. In this manner, the surface of Graphene can be restored, forming the reduced graphene oxide (rGO), which opens the door to an array of electrical applications. The preparation of dispersed Graphene allows flexible electronics to reach stability if the stability point is instantly reached. Many solvents entertain the solubility of graphene oxide. There are differences in the solubilities of graphene oxide and rGO. It depends on how much conductive can rGO surface goes. graphene oxide and rGO dispersion by removal of oxygen-containing group influences solubility.
Background
The first model of graphene oxide that became popular was proposed by Lerf-Klinowski in late 1990. The model was solely based on oxidative chemical exfoliation of Graphene with potassium permanganate (KMNO4) as an oxidizer. The carbon and hydrogen were used under nuclear magnetic resonance (SSNMR), chemical derivatization of graphite oxide, and Fourier transformed infrared spectroscopy (FTIR). Lerf-Klinowski proposed graphene oxide has two different regions: aliphatic six ringed region and aromatic benzene like rings. Later on, Dékány analyzed the structure of Go and proposed various dispersion techniques, which became the pioneer for the wide applications of dispersed Graphene. In general, Dékány integrated the process of Scholz-Boehm to support his observations. He prepared the graphene oxide with a potassium chlorate oxidation process, yet with different chemical behavior. Both the models used are considered state-of-the-art because they have much literature to be explored for applications and properties that graphene oxide can have.
Properties of Graphene Oxide
Oxygen Groups
Oxygen groups in Graphene induce the carbon layers to have a two-dimensional structure that has oxo functionalities. graphene oxide follows a non-stoichiometric general formula that goes by, CxHyOz. The value of y in the empirical formula is 0.8, while the carbon to oxygen ratio varies from 1.5 to 2.5. The compositions of atoms in graphene oxide can be examined by energy dispersive spectroscopy (EDS), high-resolution X-ray photoelectron spectroscopy XPS), and combustion elemental analysis. XPS deconvolution is mainly used to calculate the oxygen functionality in graphene oxide. There are varieties of synthetic methods to evaluate the C-O ratio. For instance, the XPS survey provides enough information about the elements of the material. The XPS survey spectrum illustrated high peaks of carbon and oxygen in graphite. All the graphene oxide materials that were tested had less than two C-O ratios. This ratio value indicates successful oxidative execution. On the contrary, the permanganate showed higher oxidation than chlorate because no Sulphur and nitrogen peaks were observed. Nonetheless, graphene oxide-HU indicated the presence of Sulphur and nitrogen.
Thermogravimetric analysis (TGA) of graphene oxide indicated a loss of mass at a temperature of 150-300 °C, which might be the result of CO, steam, and CO2 release from the reaction. At a much higher temperature of about 400-950 °C, the loss of mass was less iconic because the stable oxygen groups showed resistance. Thermogravimetric analysis (TGA) showed the lowest loss of mass even lower than graphene oxide-TO. NMR suggested that the overall oxidation of graphene oxide-HU is more than the graphene oxide-TO. The decent way for oxidizing is to assess the ratio between alcohol and epoxide using a hybridized graphitic carbon signal. Different oxygen functionalities can be differentiated using permanganate. Most importantly, the presence of carbonyl and carboxyl groups in graphene oxide underwent a unique phenomenon that was initially prepared by the Hummers and Tour method. These methods are thought to affect the pKa and pH of graphene oxide and suspended solution, respectively. Moreover, the oxygen functionalities in graphene oxide are electrochemically active and prone to the reduction process. The differences in properties of oxygen composition can give a unique electrochemical behavior to graphene oxide in permanganate solution. The C/O ratio of graphene oxide is a crucial parameter that influences the number of oxygen to be attached to graphene oxide. This addition of oxygen affects the hydrophobicity of graphene oxide, solid-state (interlayers), sheet resistance (MΩ), and chemical reactivity.
The Reduced Form of Graphene Oxide
To inspect the properties closely, researchers have tried to remove the oxygen group from graphene oxide. There are various ways to achieve reduction, such as chemicals, microorganisms, ion bombardments, microwave irradiation, heat, and electrochemistry. This selective oxygen elimination helped to produce desired physical and chemical properties in graphene oxide. Mechanical, conductivity, dispersibility, and optical properties were mainly affected by the loss of oxygen. The electrical conductivity of CrGo that is thermally treated using both Hummer and Tour and collaborators methods gave a conductivity value of 0.05 Siemens per centimeter. Moreover, graphene oxide precursor advanced the methods used in doping. The Go can be used in the doping process using ammonia as a gaseous substrate. This helps graphene oxide to possess electrochemical properties used in several devices.
To get more information about graphene oxide and reduced graphene oxide,
you can read our blog post here.
Impurities
Graphene oxide is contaminated by different hetero metallic atoms such as Co, Cr, As, Sc, Sb, Fe, and Cu. To a certain extent, these elements help insolubilization. For instance, optical emission spectrometry (OES) and ICP showed the existence of all these metals in graphene oxide. Concerning the oxidative methods used, graphene oxide-ST had slight changes in the metallic contents of the graphite. Nonetheless, the decrease in metallic contents was present in the graphene oxide synthesized from natural graphite. Atomic absorption spectroscopy (AA) revealed that over 11 metallic elements were present in graphene oxide, while in GOHU, over 15 elements were found. The decreased number of elements can be attributed to nitric acid fuming, which is very corrosive. Hence, it removes metals in the Staudenmaier method rather than using concentrated metal in Hofmann's method that disintegrates graphene oxide. Therefore, it allows graphene oxide to exhibit thermal properties.
Applications of Graphene Oxide
Stacking in Solid-State
In graphene oxide, carbon, hydrogen, and oxygen atoms are rearranged between well-organized monolayers that have a thickness of about 1nm. The thickness mainly depends upon the measures employed for thickness and the overall hydration level. graphene oxide have a flake and sheet-like structure with a size similar to that of quantum dots. Quantum dots have the same chemical composition as that of graphite oxide that has stacked layers. Oxidation methods increase the spacing between graphene layers. graphene oxide sheets can stack to form a thick structure mainly because of hydrogen bonding. Different molecules, reagents, ions, metals, and reagents can stick to the graphene oxide layers forming bi or trilayers. If there are four to ten sheets, it is called a few-layer, while if there are more than ten sheets, it is called multilamellar.
Spacers and Cross-Linkers
Graphene oxide has many applications in Nano-building. graphene oxide has membrane technology for water remediation potential and molecular separation. After drying the layers, graphene oxide can have similar properties like carbon nanotubes that have different thicknesses and directional flow. The vacuum filtration of colloidal dispersions of graphene oxide using membrane filters can help to synthesize Go sheets. For instance, by self-development of graphene oxide by hydrosol evaporation. Another alternative approach is the use of the cross-linking method in graphene oxide sheets. If graphene oxide sheets are modified with alkaline materials in trace amounts, mechanical properties can be enhanced. The divalent ions that cross-link the graphene oxide sheets can boost the chemical properties of graphene oxide.
Electric Double-Layer
Using the electric double layer (EDL) theory, graphene oxide sheets can be defined as two different layers. Different pH conditions can result in differing surface charge density and static interaction. In the presence of electric fields, the Go sheets might move around. However, this interaction will be different for different sizes, charges, and thicknesses of sheets. These effects are the prime factors in separating graphene oxide factors using electrophoretic migration. Sheet particles are crucial in adding hydrodynamic properties and dispersion stability in graphene oxide suspensions. The potential difference between stationary fluid and dispersion medium might be the result of zeta potential. Additionally, zeta potential can quantify Coulomb repulsion between two graphene oxide sheets.
Colloids
A large crystal after exfoliation and nanosheet formation results after sonicated exfoliation of Go sheets. In functional solvents, the surface energy can be asses carefully; hence, the Nanosheets are stable after re-aggregation. On the contrary, weak solvents will result in sedimentation. Well-exfoliated graphite oxide (rGO) can disperse in aqueous colloids after it is negatively charged and dispersed in water. The ionization of carboxylic acid and phenolic hydroxyl can successfully occur. This suggests that stable graphene oxide sheets can exhibit electrostatic repulsion along with hydrophilicity.
Conclusion
The vast literature and scientific research on both graphite oxide and graphene oxide are plentiful. However, there is still yet to be explored. Graphene oxide is composed of carbon, hydrogen, and oxygen and the versatile nature of these elements enable graphene oxide to be used in a wide range of application. These applications are a result of outstanding properties that these elements incorporate in graphene oxide. The current literature only supports graphene oxide synthesis by electrochemical and Hummers method for large-scale production. In a nutshell, graphene oxide is useful for fabrication due to several electrical, thermal, and mechanical properties. After filtration and casting techniques, graphene oxide can form a paper-like membrane that makes it useful in almost every electronic device in the world.
To get more information, you can visit Blografi.
References
https://pubs.rsc.org/en/content/articlelanding/2020/tc/c9tc03251g#!divAbstract
https://www.sciencedirect.com/science/article/pii/S0021979714003397
Recent Posts
-
What is the Difference Between 7075 and 6061 Aluminum Alloy?
When comparing 7075 aluminum alloy to 6061 aluminum alloy, it's essential to understand their disti …5th Apr 2024 -
Iron-Air Batteries: The Ultimate Guide
Iron-air batteries represent a significant breakthrough in energy storage technology, offering a sus …29th Mar 2024 -
Discovering the Power of 2D Materials
In material science, the discovery of two-dimensional (2D) materials represents a transformative de …22nd Mar 2024