Gadolinium Powder
As a silvery-white rare-earth metal, gadolinium (Gd) is a slightly malleable ductile chemical element categorized among the lanthanides in the periodic table of elements with the atomic weight and number of 157 and 64, respectively.
Gadolinium engages in a gradual oxidation reaction with atmospheric moisture and oxygen. The magnetic property of gadolinium is time-dependent with ferromagnetic behavior at temperatures below 20°C (Curie point) and paramagnetic behavior at temperatures above.
Introduction
Gadolinium occurs naturally only in its oxidized form along with impurities of other rare earth metals which is due to the similarities in their properties. Gadolinium was first discovered in the late 19th century using the spectroscopy technique and named after the gadolinite mineral in which it is found. Gadolinium is known to exhibit some strange metallurgical behavior as very little quantity of Gd (1%) has the ability to improve the resistance of metals such as iron and chromium against oxidization at high temperatures. In metallic form or as a salt, gadolinium is capable of absorbing neutrons with the application as neutron shielding in radiography in nuclear reactors.
Based on its three oxidation states, gadolinium forms fluorescent trivalent ions similar to most rare-earth metals. It should be noted that water-soluble gadolinium ions (III) have been proven to be toxic for human and mammals. Nevertheless, gadolinium chelates have very low toxicity since the chelating agents can carry the Gd (III) out of the body through the kidneys. Solutions containing organic gadolinium chelates and complexes are employed as intravenous medications known as gadolinium-based MRI contrast agent due to gadolinium’s paramagnetic properties.
Physicochemical Properties of Gadolinium Powder
Gadolinium forms hexagonal closed-pack crystals (α-form) at room temperatures, whereas its crystals transforms into a body-centered cubic structure (β-form) at temperatures over 1200°C. Among the 30 known isotopes of gadolinium, 157Gd possesses the highest thermal-neutron capture cross section quality as much as 259,000 barns. It should be noted that 135Xe is the only isotope to have a higher neutron capture cross-section of about 2 million barns but the problem is it is radioactive and therefore, hazardous. Gadolinium’s time-dependent ferromagnetic and paramagnetic properties demonstrate that Gd is basically a helical antiferromagnetic metal rather than a ferromagnetic material meaning it has a population of atoms which possess opposite magnetic charge within the bulk. It exhibits the magnetocaloric effect (MCE) through which its temperature rises when a magnetic field is applied with a consecutive fall in the temperature while the magnetic field is shot down. It is possible to encapsulate individual gadolinium atoms into fullerene molecules in order to incorporate them into carbon nanotubes. Although active magnetic regenerators (AMRs) use a combination of different magnetocaloric materials with low Curie temperatures in order to broaden the temperature window of the AMR from 7 to -250°C, there are attempts to consider gadolinium as the first layers regarding its unique properties. Gadolinium has particularly been studied to be applied in magnetic refrigeration since its ferromagnetic ordering transition at 20°C generates MCE at larger quantities to rich refrigeration at near-room temperature 1.
Gadolinium can engage in reactions with other Gd (III) compounds as well as carbon, arsenide, sulfur, phosphorus, selenium and boron at higher temperatures to form compounds with binary structures. Metallic gadolinium remains stable in dry air while other earth-metals aren’t stable under this condition. On the contrary, Gd tarnishes in humid atmosphere and produces gadolinium oxide (Gd2O3) which is an adhering agent. Regarding the +3 oxidization state of gadolinium, gadolinium appears to adopt this trivalent behavior is majority of its compounds. In general all gadolinium based compounds are white except for the case of its compound with iodine which is yellow. Among the halogens, its most common and available halide is gadolinium chloride. The oxide form is soluble in acids yielding salts like gadolinium nitrate. Similar to most lanthanide ions, gallium engages in reactions with coordinating ligands to form complexes with higher coordination numbers and gadolinium salts have applications in MRI (magnetic resonance imaging).
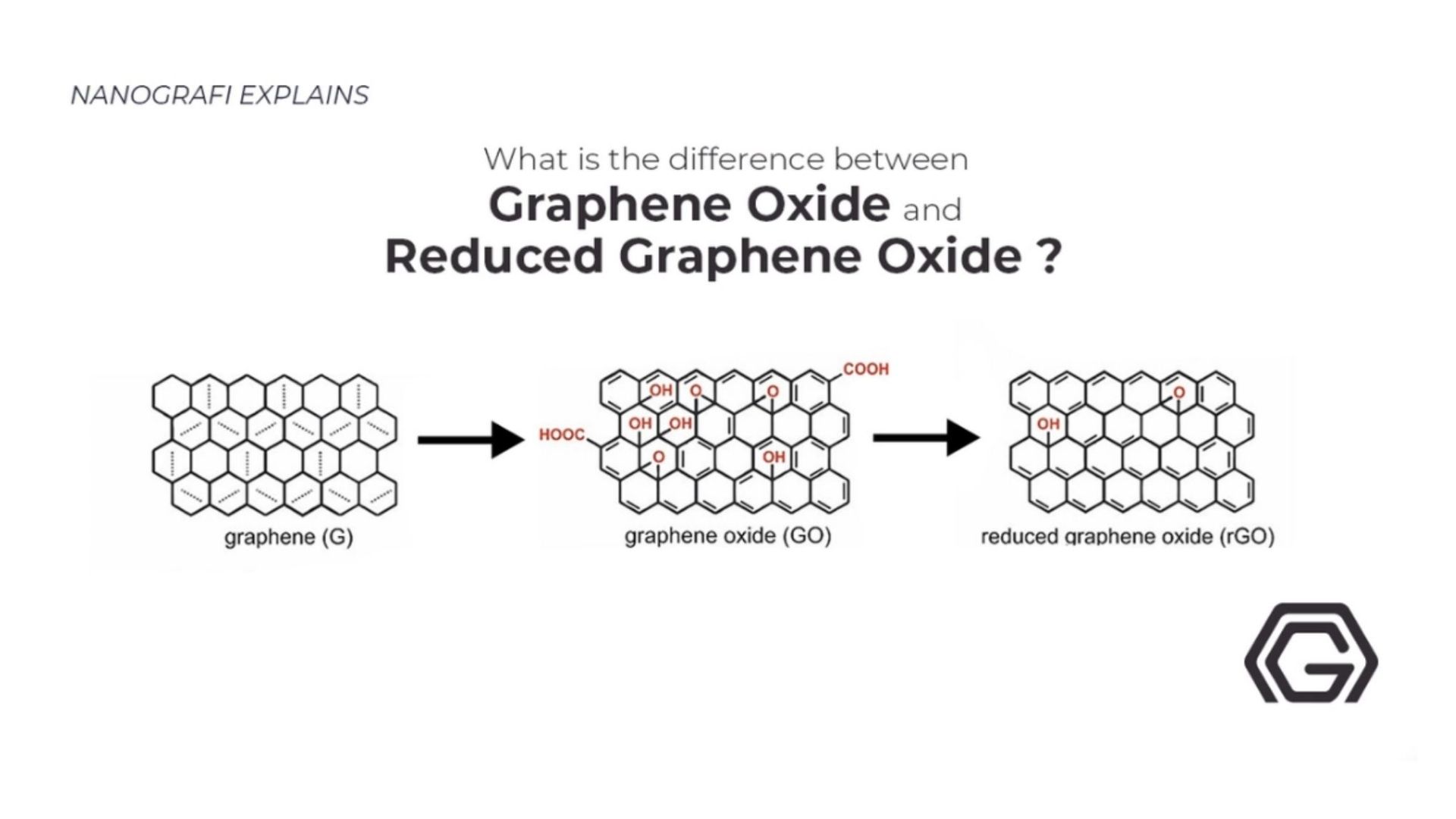
If you are interested in the difference between graphene oxide and reduced graphene oxide,
you can read our blog post here.
Synthesis and Preparation of Gadolinium Powder
In order to produce spherical metal powders as small as 200 μm to develop cryogenic magnetocaloric applications, some important factors have to be taken into consideration such as turn-around time, powder performance, expenses and cost. The turn-around time is so significant since the compositional space of materials with cryogenic magnetocaloric effect (MCE) isn’t charted so perfectly. It has been shown that atomizing gases can lead to a huge amount of powders with spherical morphologies. However, the resulting size distribution appears to be wide with the long turn-around time limitation of size tens of kilograms or more to consequently end up in a large pile of costly rare-earth metal powders with no probable application afterwards. In fact, spherical gadolinium powder is the key factor in order to achieve decent heat transmitting quality and maintain low pressure for heat transmitting fluid in the regenerator. It is a challenge to produce relatively large spherical powder of rare-earth metals to be considerably sensitive to oxygen. All in all, rotating disk atomization (RDA) technique has proved to be efficient to obtain powder with the short turn-around time in smaller quantities compared to plasma rotating electrode methods and gas atomization method. Therefore, it is appropriate to investigate and develop new magnetocaloric materials. In particular, bulk gadolinium can be atomized so satisfactory in order to obtain spherical gadolinium powder with sizes ranging from 150 to 250 μm 1. Moreover, the nanosized gadolinium powder can be achieved through condensing Gd vapor in argon atmosphere with the evaporation rate of around 10 to 33 milligram per minute. The black powder of gadolinium sized as 5 to 15 nanometers can be collected from a glass disk 2.
Applications of Gadolinium Powder
There is a fact that gadolinium has no applications in larger scales while having a lot of specific applications. As it was mentioned already, gadolinium has a high neutron cross-section and therefore, it is used to locate tumors throughout the human body in neutron therapy. It is capable of being used with neutron radiography and to shield nuclear reactors. It is also applied as a secondary and emergency shot-down in CANDU reactors as well as in nuclear marine propulsion. Considering its physical properties, gadolinium is used as a time-dependent magnetic agent. The paramagnetic ions of gadolinium can increase nuclear relaxation rates and also in magnetic resonance imaging (MRI).
Metallic gadolinium and gadolinium powder possess temperature-dependent magnetic behavior such as ferromagnetism and paramagnetism at temperatures below and above 20°C, respectively. Gadolinium also adopts metallurgic behavior advantageous to enhance metals resistance against oxidization. It is extensively used as a neutron shield and capturer with applications in nuclear technology and medicine.
To discover the latest news from nanotechnology, you can visit Blografi.
References
1. Wolf, S. et al. Synthesis and magnetic performance of gadolinium powder produced with rotating disk atomization. Powder Technol. 359, 331–336 (2020).
2. Abelson, P. H. Materials research. Science (80-. ). 235, 9 (1987).
Recent Posts
-
What is the Difference Between 7075 and 6061 Aluminum Alloy?
When comparing 7075 aluminum alloy to 6061 aluminum alloy, it's essential to understand their disti …5th Apr 2024 -
Iron-Air Batteries: The Ultimate Guide
Iron-air batteries represent a significant breakthrough in energy storage technology, offering a sus …29th Mar 2024 -
Discovering the Power of 2D Materials
In material science, the discovery of two-dimensional (2D) materials represents a transformative de …22nd Mar 2024