Ferrite Nanoparticles and Their Applications
Nanoparticles are one of the most reliable and excellent sources for carrying out scientific and industrial applications while keeping up with the advanced systems of technologies. Ferrite group nanoparticles too are one of the major groups of nanoparticles that play their role in enhancing and uplifting the industries of the entire world.
They are divided into different categories to make the understanding of each category more effective and efficient. Cobalt ferrite group is the most renowned and common one therefore, its applications are quite vast too, a few of which are explained in this article. However, all the other categories of the ferrite group are highly effective and usable too and are taking the industries to new heights through their characteristics. For an in-depth exploration of the cutting-edge advancements and the latest research in nanotechnology, visit Blografi.
Introduction
For enhanced technologies, the progress of functional nanomaterials is very significant for applications in science and industry. Natural resources are being used as the starting compounds to produced nanomaterials. The high-selling natural and functional materials are currently being explored by researchers for numerous specific applications. For instance, there were reports on silica sand’s usage from Tanah Laut, Kalimantan, Indonesia, as a raw material to produce zircon, pure SiO2, and zirconia with high phase purity and the size of their crystals was in the nanometer range.
Besides, natural iron sand is being explored as a starting material as it manufactures magnetite (Fe3O4) nanoparticles as magnetic fluid (ferrofluid), magnetic coating, and magnetic gel (ferrogel) for tissue engineering, applications in biomedicine, and radar-absorbing materials.
What is Ferrite Oxide (Fe3O4)?
Fe3O4, one of the magnetic particles, also called iron oxide, can be attained from the natural iron sand. Chemical and mechanical processes are used to separate it from the impurities of natural iron sand. Naturally, Iron sand has more than 90% weightage of Fe3O4 particles. FeCl3.6H2O and FeCl2.4H2O are the two commercial raw materials that are generally used in synthesizing Fe3O4.
Techniques of co-precipitation, hydrothermal, and sol-gel are the commonly utilized methods of synthesis. There has been a wide application of the addition of templates or surfactants for producing homogeneous nanoparticles with particular morphologies and sizes as the Fe3O4 nanoparticles agglomerates among the particles. For the past few years, the main topic of the research has been the preparation of Fe3O4 nanoparticles from iron sand. For instance, doping of Zn and Mn on Fe3O4 makes Fe3O4 superparamagnetic, therefore making it capable of being applied in applications in biomedicine.
Dielectric and Magnetic Properties
The size of the particle and doping influences the BiFeO3 nanoparticle's dielectric and magnetic characteristics. The duration of calcination, temperature, and the method of synthesis has an effect on those characteristics. According to studies, all of the magnetic parameters, for instance, the saturation magnetization increases when there is a decrease in the size of the particle. At room temperature, weak ferromagnetism is possessed by those BiFeO3 nanoparticles whose size is less than 100 nm. Such ferromagnetic behaviour of the BiFeO3 nanoparticles is because of the oxygen vacancies being present in the BiFeO3 system.
Till now, numerous researches have examined the BiFeO3 nanoparticle's doping effects with various advanced methods for enhancing their performance. The magnetization can also be enhanced by doping probably because of the presence of impurity phase in the BiFeO3 system, destruction of the spin cycloid structure, distortion of Fe and O bonding, increasing the Dzyaloshinskii-Moriya (DM) interaction’s effect, and increasing distortion of the local structure
Moreover, the conditions of the atmosphere during the power syntheses strongly determine the dielectric characteristics of the pure BiFeO3 phase. A higher spontaneous polarization was found by Liu et al., along with the lower breakdown field that’s based on the polarization-electrical field (P-E) hysteresis loops in the samples that are annealed in the atmospheres of N2 and H2. According to this article, the sol-gel method synthesized BiFeO3 nanoparticles by utilizing natural iron sand which is one of its raw materials and calcined in the air atmosphere. Later, there were focused investigations on the dielectric and ferroelectric characteristics of the Ni- and Pb-doped BiFeO3 nanoparticles.
Different Forms of Ferrite Oxide
At high temperatures, the most stable iron oxide is Hematite (α-Fe2O3). Commonly, the α-Fe2O3 comes from iron rust as it is a dominant corrosion product of an iron alloy or iron metal. Generally, several methods like co-precipitation and hydrothermal technique, have taken place for successfully producing α-Fe2O3 nanoparticles by utilizing commercial raw metals, for instance, FeCl3.6H2O and Fe(NO3)39H2O. According to research, the morphology and size of the particle, along with its optical band gap can be influenced by the concentration of Fe3+ ions that is utilized in the synthesis of α-Fe2O3 nanoparticles. Those α-Fe2O3 nanoparticles which have 8 nm particle size have superparamagnetic characteristics with comparatively high magnetization at room temperature. Thus it’s a possibility for it to be implemented for spintronic and biomedical applications.
There are different categories in which ferrite groups are divided, a few of them are explained below:
Calcium Ferrite
Soft ferromagnetism is exhibited by calcium ferrite compounds, thus it can be utilized in the calcium ferrite/graphite nanocomposites for radar-absorbing materials. Magnetic characteristics are possessed by calcium ferrite nanoparticles, they can be compared to strontium ferrite and barium ferrite, and are also called M-type hexaferrite for microwave-absorbing applications. For applications in biomedicine and as a targeted drug delivery agent, superparamagnetic behaviour should be possessed by calcium ferrite.
As compared to other ferrites like MFe2O4 (M can be Cu, Ni, Mn, and Zn), one of the environmentally friendly and biocompatible materials is CaFe2O4 because of the utilization of calcium instead of heavy metals. In addition, a specific application is possessed by Ca2Fe2O5 with the brown millerite structure as a p-type thermoelectric device because of this compound’s unique electrical characteristics. The electrochemical activity may be improved by the oxygen deficiencies in the Ca2Fe2O5 crystals. The magnetic characteristics of CaFe4O7 are not explored yet. As compared to other calcium ferrites that are discussed here, the CaFe4O7 nanoparticles were made by mixing CaCO3 from natural limestone and Fe2O3 from natural iron sand.
Bismuth Ferrite
At room temperature, Bismuth ferrite (BiFeO3) is one of the multiferroic systems which displays magnetic-electric coupling. The chemical formula of ABO3 and a perovskite structure is possessed by the multi-ferroic material. The magnetic, electronic, and structural characteristics may get affected by the presence of oxygen vacancies, the cation nonstoichiometry, and the type of A and B sites. With space group R3c, BiFeO3 crystallizes in a distorted rhombohedral perovskite. It has a Néel temperature of 640 K and a high Curie temperature of 1100 K, respectively.
Attaining BiFeO3's pure phase is difficult because the phase formation’s kinetics results in the production of secondary phases, for instance, Bi2Fe4O9 (mullite) and Bi25FeO40 (sillenite). Numerous techniques like sol-gel, hydrothermal, and chemical coprecipitation methods are reported to synthesize a single phase of BiFeO3 because those methods have an idea of achieving the BiFeO3's single phase with low temperature, and a cost-effective, yet simple route. According to Wang et al., at 425 C, the BiFeO3 starts forming by the low-heating temperature solid-state precursor method with impurity phases of about 30%.
To get more information about nanoparticles, you can read our blog post here.
Cobalt Ferrite Nanoparticles
With the octahedral sites (B sites) being occupied by all or most of the Co+2 ions and the tetrahedral sites (A sites) and B sites being occupied by the Fe+3 ions, cobalt spinel ferrite (CoFe2O4) is an inverse spinel ferrite in this category (spinel ferrites). The reason for cobalt spinel ferrites (CoFe2O4) gaining so much attention is because of their excellent chemical and good mechanical stabilities at room temperature, their moderate saturation magnetization, high coercivity, and their strong anisotropy. The chemical and mechanical stabilities of cobalt spinel ferrites are very different from their bulk counterparts. Due to all of these properties, cobalt ferrites have been broadly utilized in biotechnology, catalysis, biomedical, magnetic drug delivery, solar cells, magnetic cards, recording devices, and sensors. CoFe2O4 nanoparticles should have high magnetization values and narrow size distribution for catalysis applications.
In comparison
Despite having some drawbacks, γ-Fe2O3 and Fe3O4 can be made easily, and are more famous than CoFe2O4. They have also been utilized, efficiently in many organic reactions. For instance, γ-Fe2O3 is thermally unstable and Fe3O4 is fairly reactive to oxidative and acidic environments. Also, comparatively, excellent chemical stability is possessed by the CoFe2O4 nanoparticles. A series of factors strongly determine the properties of CoFe2O4 nanoparticles. The factors are, for instance, their chemical composition, and their microstructural properties. The shape and size of the particle are controlled and monitored in the fabrication processes.
Preparation of CoFe2O4 Nanoparticles
A different number of preparation methods, for instance, combustion methods, electrochemical method, co-precipitation, solvothermal method, hydrothermal synthesis, sol-gel techniques, and microemulsions, have been used and explored for producing CoFe2O4 nanoparticles. But the cobalt ferrite nanoparticles are produced in the required or desired microstructures and sizes in most methods of preparation, making it difficult to implement them on a broader scale because of their potential danger to the environment, toxic by-products, toxic reagents, long reaction times, high reaction temperatures, and complicated and expensive procedures.
Out of all the preparation methods, the easy ones are hydrothermal and co-precipitation techniques as they are cheap and versatile too. Two factors are significant in the hydrothermal process. One is the hydrothermal synthetic method’s optimization, and the other is cobalt ferrite nanoparticles’ surface modification or ion doping. Ion doping can cause many effects, for instance, it can result in cation redistribution, change in lattice strain, change in the size of the grain, a disorder in the structure, along with the effects in chemical and physical characteristics of the cobalt ferrite nanoparticles.
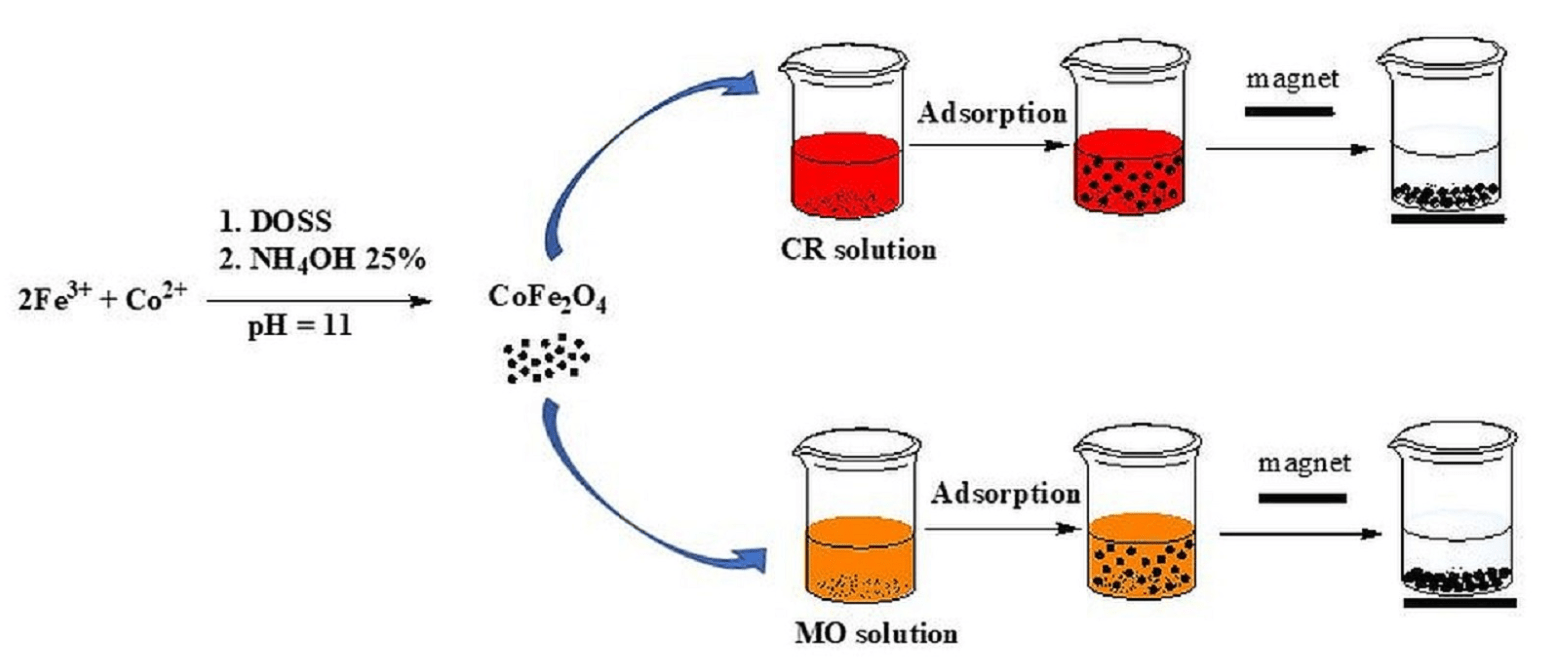
Figure 1. Facile synthesis of cobalt ferrite (CoFe2O4) nanoparticles.
Applications of Cobalt Ferrite Group Nanoparticles
Biosensors
CoFe2O4 NPs are widely studied in biosensing applications because of their ability to align themselves with the external magnetic field. They can either be dispersed in the solution of desired analytes or immobilized over a transducer surface for further analyte detection. Usually, the NP is coated with a targeting moiety such as an antibody that interacts with the analyte of interest, and the transducer produces a signal that is sensed by the detector.
Biosensors for CEA detection with high sensitivity are being researched actively to aid in early and more reliable cancer diagnosis. CEA is a glycoprotein overexpressed in cancer and commonly used to follow up patients with colorectal cancer. A quartz crystal microbalance (QCM) based immunosensor was fabricated by Chen et al. with CoFe2O4@SiO2 immobilized on the surface that is conjugated with an anti-CEA antibody for piezoelectric immune-sensing of CEA antigen. For a 25-min incubation time, the detection limit was reported to be 0.5 ng/ml, the signal-noise ratio was 3 and the sensor showed a wide linear range (2.5–55 ng/ml). N-terminal pro-brain natriuretic peptide (NT-proBNP) is an effective diagnostic and prognostic marker for heart failure.
A novel sandwich-type immunosensor was developed by He et al. involving antibody conjugated gold functionalized CoFe2O4 NPs (CoFe2O4@AuNPs) for the detection of NT-proBNP. The immunosensor exhibited a wide detection range (1 fg ml-1 to 1 ng ml-1) and high sensitivity (detection limit – 0.75 fg ml-1) with good reproducibility.
Magnetic Separation & Purification
CoFe2O4-NPs on account of magnetic susceptibility have been utilized for magnetic extraction, separation, and purification of various substances including pollutants and toxins from environmental and biological samples. In addition to that due to the high specific surface area and porous structure, these NPs possess high adsorption efficiency making them interesting adsorbent materials for separation and purification applications. Bilirubin (BR) is a yellow-orange breakdown product of heme; excess BR deposits in various tissues are observed in cases of hyperbilirubinemia, jaundice, and neurotoxicity. Therefore, the use of adsorptive materials for the reduction of BR levels in the body is promising.
Rakshit et al. used nano-hollow spheres of CoFe2O4-NPs and silica-coated CoFe2O4 core@shell NPs (CoFe2O4@SiO2) for adsorptive degradation of BR. The authors have reported an excellent efficiency of the NPs with 98% BR adsorption taking place within 8 min in the case of nano-hollow spheres and 90% within 20 min for CoFe2O4@SiO2 NPs of diameter 350 nm, suggesting the use of these NPs for decreasing BR levels in therapeutic applications against hyperbilirubinemia. In another study, Pal et al. synthesized Na-tartrate surface-functionalized CoFe2O4-NPs (T-CoFe2O4) for degradation of BR. The T-CoFe2O4 exhibited multicolor fluorescence emission spectra giving rise to cyan, green, and red at excitation wavelengths of 365, 436, and 546 nm. The T-CoFe2O4 NPs facilitated photodegradation of BR within 50 min yielding the photooxidation product methyl-vinyl maleimide. The reported NPs for BR degradation were recyclable and can be used up to five times. Thus, the NPs exhibited multifunctional properties such as superparamagnetism and intrinsic fluorescence.
To get more information about the application areas of nanoparticles, you can read our blog post here.
Drug Delivery
CoFe2O4-NPs are promising materials as nanocarriers for drug delivery applications with encouraging results and studies that have reached clinical trials in the past decade. This stems from three interesting properties namely: a high surface area for effective drug loading; feasibility to produce a wide variety of multifunctional nanocomposites and ability to be controlled via an externally applied magnetic field for magnetic targeting. Magnetically driven dandelion pollen-like CoFe2O4 nanostructures were proposed as a controlled drug release system for doxorubicin (DOX) delivery. The nanostructures possessed 88.6% loading capacity, loading content of 118 mg of DOX/g of the CoFe2O4 microspheres, and ∼55% drug release under alternating magnetic field (AMF) field at 8 h.
What do you know about nanomedicine and nanomedicines? Learn now.
Imaging
MNPs have been in the limelight as contrast agents for MRI with improved contrast and biocompatibility compared with conventional agents such as gadolinium. Higher the longitudinal and transverse relaxivity (r1 & r2, respectively) of a given contrast agent, the better is the resulting contrast. Existing contrast agents have a short half-life in vivo, and therefore cannot give prolonged information for prognostic assessment of a given treatment. For a long-term MR contrast platform, Kim et al. designed a homogenous magnetic hydrogel system using CoFe2O4 NPs containing thermosensitive poly (organophosphate) hydrogels.
With a high relaxivity value (159.74 mM/s), these NPs showed good potential to be long-term negative contrast MR agents. F344 rats were used for in vivo imaging studies, and the hydrogels were injected into the brains of rats. After 4 days of injection, the authors observed a strong-negative MR T2 contrast on the right-hand side of the frontal cortex region of the rat brain. The negative contrast did not blur out or spread till 32 days post-surgery. The authors suggested that these hydrogels have potential as long-term theranostic agents especially in the case of brain tumors.
Hyperthermia
MHT is a therapeutic intervention wherein MNPs are administered, and an external radiofrequency field is applied to MNPs that are targeted to the tumor site. Application of radiofrequency field increases the temperature of MNPs thereby increasing the temperature of MNP containing cells as well, resulting in cell death by thermo-ablation. By far, MHT is the most widely studied biomedical application utilizing CoFe2O4 NPs. This is mainly because the high magnetic anisotropy of CoFe2O4 NPs provides better heating efficiency at very small sizes as compared with IONPs.
Before reviewing the literature for MHT studies involving CoFe2O4 NPs, some important magnetic parameters that greatly influence the heating efficiency must be acknowledged. The specific absorption rate (SAR) or specific loss power is defined as the amount of energy dissipated by the NPs per unit mass of the particles per unit time. Therefore, measurement of specific loss power indicates an estimation of the increase in temperature of the NPs in response to the applied field and hence the hyperthermic efficiency of the NPs.
Conclusion
Cobalt ferrite nanoparticles have gained popularity due to the outstanding characteristics that they hold which enables them to be utilized in various fields and serve the purpose. The diversity in ferrite group nanoparticles is due to the sub-categories it has been divided to accommodate all the elements that can combine with it. However, all of these compounds are outstanding and very beneficial for the industries.
References
Antisymmetric exchange - Wikipedia. (n.d.). Retrieved February 13, 2024, from https://en.wikipedia.org/wiki/Antisymmetric_exchange
Baqiya, M. A., Asih, R., Ghufron, M., Mastuki, Retnowati, D. Y., Triwikantoro, & Darminto. (2019). Ferrite-Based Nanoparticles Synthesized from Natural Iron Sand as the Fe3+ Ion Source. Nanocrystalline Materials. https://doi.org/10.5772/INTECHOPEN.88027
Cobalt ferrite nanoparticles (CoFe2O4 MNPs) as catalyst and support: magnetically recoverable nanocatalysts in organic synthesis. (n.d.). Retrieved February 13, 2024, from https://www.degruyter.com/document/doi/10.1515/ntrev-2017-0138/html
Kazemi, M., Ghobadi, M., & Mirzaie, A. (2018). Cobalt ferrite nanoparticles (CoFe2O4 MNPs) as catalyst and support: Magnetically recoverable nanocatalysts in organic synthesis. Nanotechnology Reviews, 7(1), 43–68. https://doi.org/10.1515/NTREV-2017-0138/ASSET/GRAPHIC/J_NTREV-2017-0138_EQ_14.JPG
Nanomedicine and Nanodrugs - Nanografi Blog - Nanografi Nano Technology. (n.d.). Retrieved February 13, 2024, from https://nanografi.com/blog/nanomedicine-and-nanodrugs/
Iron Oxide Nanoparticles/Nanopowder and Applications - Nanografi Nano Technology. (n.d.). Retrieved February 13, 2024, from https://nanografi.com/blog/iron-oxide-nanoparticlesnanopowder-and-applications/
Simonescu, C. M., Tătăruş, A., Culiţă, D. C., Stănică, N., Butoi, B., & Kuncser, A. (2021). Facile Synthesis of Cobalt Ferrite (CoFe2O4) Nanoparticles in the Presence of Sodium Bis (2-ethyl-hexyl) Sulfosuccinate and Their Application in Dyes Removal from Single and Binary Aqueous Solutions. Nanomaterials 2021, Vol. 11, Page 3128, 11(11), 3128. https://doi.org/10.3390/NANO11113128
Structural, Electrical, Dielectric, and Magnetic Properties of Cd2+ Substituted Nickel Ferrite Nanoparticles. (n.d.). Retrieved February 13, 2024, from https://www.hindawi.com/journals/jnp/2016/4709687/
Recent Posts
-
Nanocomposites in Food Packaging
The utilization of nanocomposites in food packaging represents a significant advancement in the fiel …19th Apr 2024 -
What is the Difference Between 7075 and 6061 Aluminum Alloy?
When comparing 7075 aluminum alloy to 6061 aluminum alloy, it's essential to understand their disti …5th Apr 2024 -
Iron-Air Batteries: The Ultimate Guide
Iron-air batteries represent a significant breakthrough in energy storage technology, offering a sus …29th Mar 2024








