Boron Carbide Nanoparticles History, Properties, Application
Boron Carbide Nanoparticles are significant materials produced through nanotechnology advancements. They stand out for high hardness, low density, and excellent thermal conductivity. Primarily used in defense for armor-making and nuclear energy applications, they also hold potential in medicine, electronics, and aerospace industries.
The boron carbide nanoparticles are of approximately 40 nm in diameter. It is used across a number of industries due to its hardness, unique interaction with neutrons, and basic properties. Boron carbide nanoparticles have experienced extensive research from engineers and researchers. The mechanical properties of Boron Carbide make it useful in various applications. This article is focused on Boron Carbide Nanoparticles, its history, properties, manufacturing and applications in different sectors. At Nanografi, we strive to provide you with detailed information about our products while offering you high-quality products using cutting-edge technology.
Introduction
Boron carbide (B4C) is often named as “Black diamond”. It is a black crystalline solid almost as hard as diamond. The compound contains 1 Carbon atom and 4 Boron atoms. It is grayish black in color and usually a very hard ceramic material. It is to be noted that Carbon and Boron are placed adjacent to each other in the periodic table, having atomic numbers like 6 and 5, respectively.
Boron carbide nanoparticles are known for their high strength, durability, and wear resistance, making them highly desirable for a variety of applications. Due to their exclusive mechanical properties and hardness, they are used in the production of advanced ceramics, especially as reinforcements in composite materials.
Additionally, these nanoparticles find application in the field of nanotechnology, where their unique properties are utilized for various purposes. For example, they may be employed in nanoelectronics, nanosensors, and as catalysts for specific chemical reactions.
History of Boron Carbide (B4C) Nanoparticles
The history of Boron Carbide nanoparticles begins with the discovery of a compound composed of boron and carbon atoms. It was first synthesized by French chemist Henri Moissan in 1899. In the early years, it found applications in industries due to its high mechanical and thermal properties.
With the advancement of nanotechnology, the study of materials like Boron Carbide at the nanoscale became possible, leading to increased research on Boron Carbide nanoparticles. These nanoparticles have been synthesized using methods such as chemical vapor deposition (CVD), ball milling, and laser ablation.
Boron Carbide nanoparticles exhibit exceptional hardness, high thermal stability, and excellent mechanical properties, making them potential candidates for reinforcement in composite materials, neutron radiation shielding, and armor applications. Research on Boron Carbide nanoparticles is still ongoing, and scientific knowledge continues to evolve.
8 Properties of Boron Carbide (B4C) Nanoparticles
The properties of Boron Carbide Nanoparticles are described below in detail:
1. Molar Weight of B4C Nanoparticles
The molar weight of the compound is 55.255 g/mol, whereas the density is 2.52 g/cm3.
2. Crystal Structure of B4C Nanoparticles
Crystal structure of boron carbide is characterized by a combination of covalent and ionic bonding. The basic structural unit in boron carbide is the B11C icosahedron, which consists of twelve vertices (atoms) that form an icosahedral cage-like structure. Within this cage, there are three boron atoms and one carbon atom positioned at the vertices of the icosahedron.
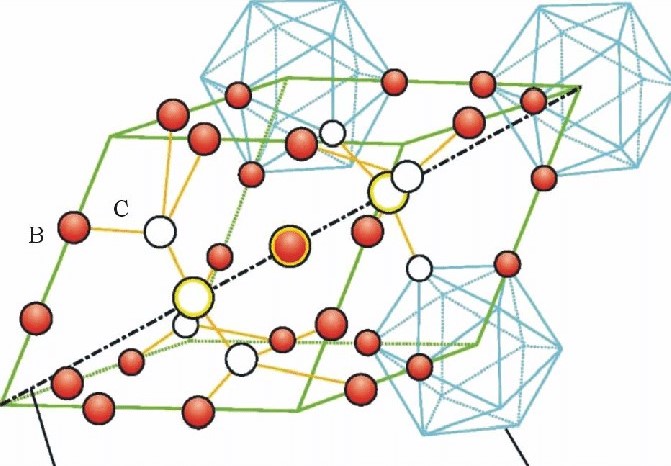
Figure 1: Structure of boron carbide.
3. Particle Size Distribution and SSA of B4C Nanoparticles
It has a thin range of particle size distribution, larger specific surface area, and good purity. The Specific Surface Area of Boron carbide Nanoparticles is greater than 42 m2/g.
4. Melting Point of B4C Nanoparticles
The melting point of Boron carbide Nanoparticles is approximately 2350 °C.
5. Boiling Point of B4C Nanoparticles
The boiling point of Boron carbide Nanoparticles is approximately 3500 °C and the hardness is up to 9.3, whereas the bending force is greater than 400 MPa.
6. Reactivity and Stability of B4C Nanoparticles
Boron carbide Nanoparticles do not react with alkali or acid solution. The compound is usually resistant to high temperatures, and it has anti-oxidation properties, high strength, high hardness, high crushing efficiency, high elastic modulus, good self-lubricating characteristics, and larger wear resistance.
The nanoparticles of Boron carbide have a higher cross section of thermal neutron capture, with good anti-radiation performance and excellent neutron absorption properties.
7. Color of B4C Nanoparticles
Boron carbide Nanoparticles are commonly found in Black color.
8. Other Properties of B4C Nanoparticles
Its purity is up to 99%.
The size of the Boron carbide Nanoparticles is around 50 nm.
Production Process of Boron Carbide Nanopowder
Boron carbide nanopowder is a high-performance material that finds applications in various fields, including ceramics, nuclear industries, and abrasives. The production process of boron carbide nanopowder typically involves two main methods: chemical vapor deposition (CVD) and ball milling.
Chemical Vapor Deposition (CVD) Method:
CVD is a popular technique for producing boron carbide nanopowder due to its ability to control particle size and purity. The process involves the following steps:
a. Precursor Preparation: A gaseous boron-containing compound, such as boron trichloride (BCl3), and a carbon-containing gas, like methane (CH4), are selected as precursors. These precursors are typically used in a vaporized form.
b. Reactor Setup: A reactor chamber is used to carry out the CVD process. The chamber is usually made of quartz or another suitable material that can withstand high temperatures.
c. Reaction: The vaporized boron and carbon precursors are introduced into the reactor chamber at elevated temperatures, typically between 1200 to 1600°C. In the presence of a suitable catalyst or under controlled conditions, a chemical reaction takes place, leading to the formation of boron carbide nanoparticles.
d. Collection: The newly formed boron carbide nanoparticles are collected on a substrate or filter within the reactor chamber.
e. Purification and Characterization: The collected nanoparticles may undergo further purification steps to remove any impurities. Characterization techniques such as scanning electron microscopy (SEM), X-ray diffraction (XRD), and transmission electron microscopy (TEM) are used to assess the size and quality of the produced nanoparticles.
Visit Blografi to read our article about the production of graphene through the Chemical Vapor Deposition technique.
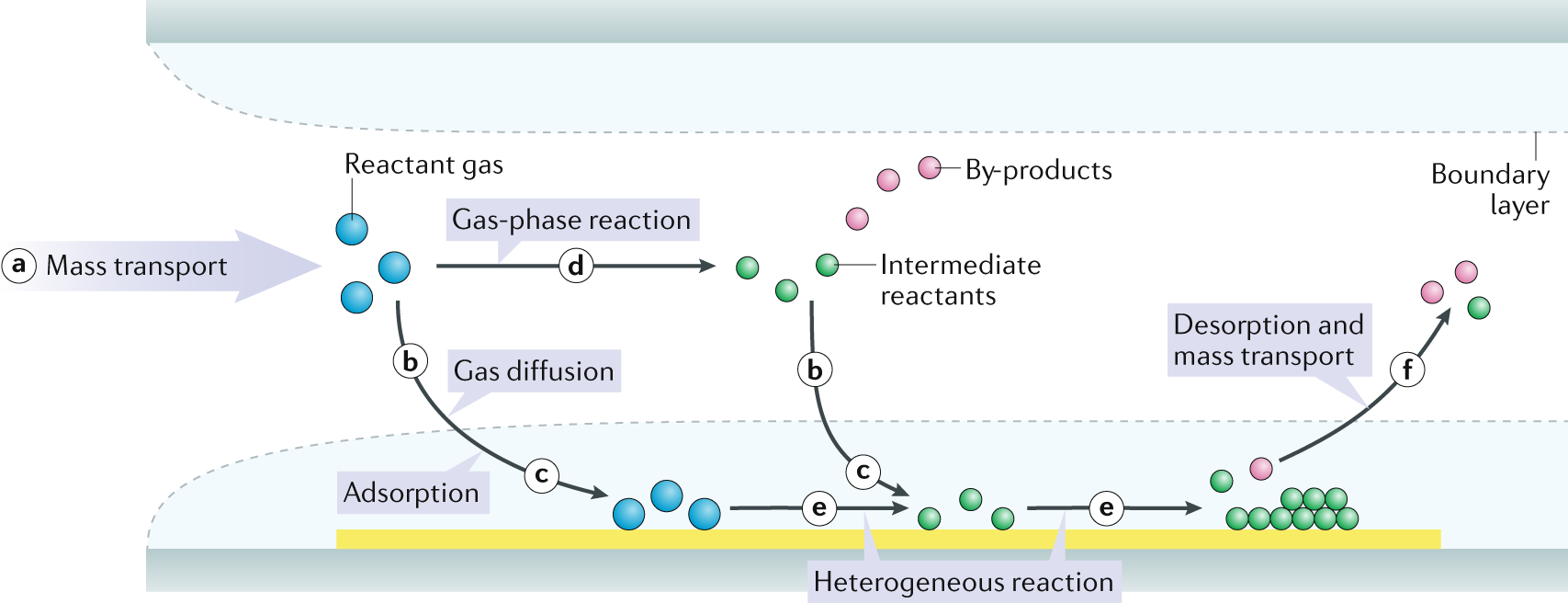
Figure 2: Chemical vapour deposition.
Ball Milling Method:
Ball milling is another common technique used for the production of boron carbide nanopowder. The process involves mechanical grinding and comminution of bulk boron carbide material into nanoparticles. The steps include:
a. Raw Material Preparation: Boron carbide in bulk form is crushed into small particles or powder to serve as the starting material.
b. Milling: The prepared boron carbide powder is loaded into a ball mill, which is a rotating cylindrical vessel containing grinding media such as balls made of hardened steel or ceramic materials.
c. Milling Process: The ball mill is operated for a specific duration and at a controlled speed, during which the collision between the grinding media and the boron carbide particles reduces the size of the particles to the nanoscale.
d. Post-Milling Treatment: After milling, the resulting nanopowder may undergo post-treatment processes to remove any impurities or surface contaminants.
e. Characterization: As with the CVD method, the final boron carbide nanopowder is characterized using various techniques to confirm its particle size, distribution, and purity.
Both the CVD and ball milling methods have their advantages and limitations. CVD allows precise control over particle size and purity but can be more complex and expensive. On the other hand, ball milling is relatively simpler and cost-effective but may have limitations in achieving extremely uniform nanoparticle sizes and higher purity levels. The choice of the production method depends on the specific requirements and applications of the boron carbide nanopowder.
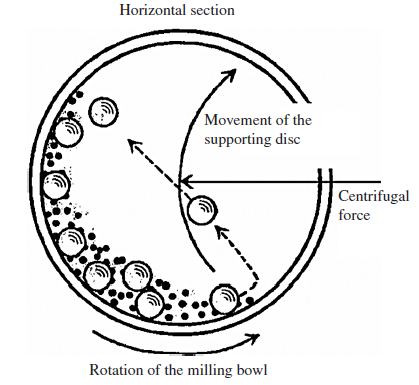
Figure 3: Schematic view of motion of the ball and powder mixture.
9 Applications of Boron Carbide Nanoparticles
Boron Carbide (B4C) nanoparticles find various applications across different industries due to their unique properties. Some of the key applications of Boron Carbide nanoparticles are as follows:
- Armor and Ballistic Protection: Boron Carbide nanoparticles are commonly used in the manufacturing of tank armors and bulletproof vests due to their exceptional hardness and low density. They provide effective protection against ballistic and projectile threats.
- Nuclear Industry: Boron Carbide nanoparticles have significant applications in the nuclear sector. They are used in control rods and shutdown pallets for nuclear reactors. The nanoparticles' ability to capture neutrons makes them useful for neutron detection. Additionally, their thermal shock resistance and thermal conductivity make them suitable for use in nuclear fusion reactors. Boron Carbide nanoparticles also help absorb neutrons without generating long-lived radiation.
- Cutting and Grinding Tools: The extreme hardness of Boron Carbide nanoparticles makes them ideal for cutting and grinding tools. Their stability ensures that they do not interact with alkali acid solutions, making them durable and reliable for various cutting and grinding applications.
Read our blog to learn about the use of graphene-based materials in anti-corrosion coatings.
- Polishing and Lapping: Due to their aggressive nature and hardness, Boron Carbide nanoparticles are utilized in polishing and lapping applications, especially in the manufacturing of precision components and optics.
- Coatings: Boron Carbide nanoparticles are used for coating various materials, such as blade tools, to enhance their wear resistance and protect against thermal shocks. They also find applications in forming thin films on surfaces, like ultra-high-density disk drives.
- Advanced Technology Applications: Boron Carbide nanoparticles are essential components in ceramic armor applications for personal use and equipment. They are also used as abrasives for grinding and polishing media, blasting nozzles, ceramic bearings, and semiconductor applications for dielectric barriers.
- Aerospace Industry: In the aerospace sector, Boron Carbide nanoparticles are used as a replacement for Be (beryllium) alloys. Their low density, high stiffness, and low thermal expansion make them valuable materials for aerospace applications.
To get more information about use of lithium-ion battery for aerospace industry, you can visit Blografi.
- Reinforcement in Composite Materials: Boron Carbide nanoparticles are used as reinforcement materials in plastic matrix composites to enhance their mechanical properties and overall performance.
- High-Temperature Electronic Devices: Boron Carbide nanoparticles find applications in high-temperature electronic devices, particularly thermoelastic devices. They also serve as dielectric barriers in semiconductor applications.
Conclusion
Boron Carbide nanoparticles (B4C) are remarkable materials with a rich history of research and development. These nanoparticles exhibit exceptional hardness, low density, and excellent thermal conductivity, making them highly sought after for various applications in different industries.
Their applications extend to nuclear industry, aerospace, and high-temperature electronic devices, showcasing the versatility and importance of these nanoparticles in modern technological advancements.
To access boron carbide nanoparticles with superior thermal conductivity and high hardness, visit Nanografi.
References
Boron carbide - Wikipedia. (n.d.). Retrieved March 5, 2024, from https://en.wikipedia.org/wiki/Boron_carbide
Boron Carbide: Structure, Properties, & Preparation. (n.d.). Retrieved March 5, 2024, from https://collegedunia.com/exams/boron-carbide-chemistry-articleid-5812
Carlsson, J. O., & Martin, P. M. (2009). Chemical Vapor Deposition. Handbook of Deposition Technologies for Films and Coatings: Science, Applications and Technology, 314–363. https://doi.org/10.1016/B978-0-8155-2031-3.00007-7
Chemical Vapor Deposition CVD Graphene - Nanografi Nano Technology. (n.d.). Retrieved March 5, 2024, from https://nanografi.com/blog/chemical-vapor-deposition-cvd-graphene/
High Energy Ball Mill Equipment and Its Application Areas - Nanografi Nano Technology. (n.d.). Retrieved March 5, 2024, from https://nanografi.com/blog/high-energy-ball-mill-equipment-and-its-application-areas/
Marsh, R. A., Vukson, S., Surampudi, S., Ratnakumar, B. V., Smart, M. C., Manzo, M., & Dalton, P. J. (2001). Li ion batteries for aerospace applications. Journal of Power Sources, 97–98, 25–27. https://doi.org/10.1016/S0378-7753(01)00584-5
Motion of the ball and powder mixture during milling (source: [10]). | Download Scientific Diagram. (n.d.). Retrieved March 5, 2024, from https://www.researchgate.net/figure/Motion-of-the-ball-and-powder-mixture-during-milling-source-10_fig2_321698733
Nanomaterials for Excellent Ballistic Protection - Nanografi Nano Technology. (n.d.). Retrieved March 5, 2024, from https://nanografi.com/blog/nanomaterials-for-excellent-ballistic-protection/
Sun, L., Yuan, G., Gao, L., Yang, J., Chhowalla, M., Gharahcheshmeh, M. H., Gleason, K. K., Choi, Y. S., Hong, B. H., & Liu, Z. (2021). Chemical vapour deposition. Nature Reviews Methods Primers 2021 1:1, 1(1), 1–20. https://doi.org/10.1038/s43586-020-00005-y
The Use of Graphene-Based Materials in Anti-Corrosion Coatings - Nanografi Nano Technology. (n.d.). Retrieved March 5, 2024, from https://nanografi.com/blog/the-use-of-graphenebased-materials-in-anticorrosion-coatings/
Recent Posts
-
Nanocomposites in Food Packaging
The utilization of nanocomposites in food packaging represents a significant advancement in the fiel …19th Apr 2024 -
What is the Difference Between 7075 and 6061 Aluminum Alloy?
When comparing 7075 aluminum alloy to 6061 aluminum alloy, it's essential to understand their disti …5th Apr 2024 -
Iron-Air Batteries: The Ultimate Guide
Iron-air batteries represent a significant breakthrough in energy storage technology, offering a sus …29th Mar 2024






