Explained: Metal Organic Frameworks - Nanografi Blog
Metal organic frameworks have attracted great attention in the last decades. Their tunable porous crystalline structure is nothing like previously constructed materials. This novel structure provides various different application opportunities for the industry. Riveting magnetic, optical, thermal, catalytic, and adsorption properties are attained through different synthesis methods and conditions. Important application areas of MOFs can be listed as gas adsorption, separation and storage, heterogeneous catalysis, energy storage, proton conductivity, sensors, and biomedicine.
Check Our Other Article About Metal Organic Frameworks
Technology has concurred the macro-scale world and provided great opportunities for humanity. It is thriving to go further, provide many other answers and solutions. However, the basis of this progress lies in nanometer-scale structures and dynamics. Natural processes show incredible behaviors on this scale and inspire scientists to develop structures with high sensitivity, selectivity, and efficiency. In order to achieve this goal, one must be able to structure matter at the nanometer scale. Metal-organic frameworks (MOFs) present a good example of these nanoscale structures.
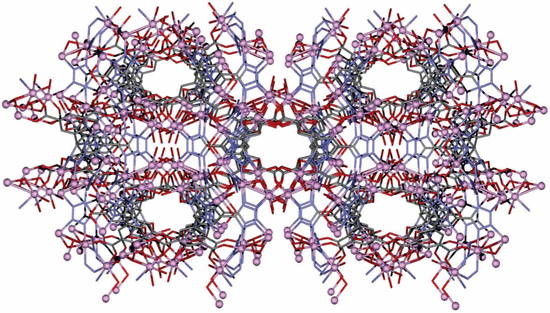
Metal organic frameworks are a class of crystalline porous materials. MOF structures consist of positively charged metal centers and organic linkers between these metal clusters. Metal clusters in the MOFs structures are also commonly called secondary building units (SBUs) in chemistry. Arrangement of SBUs and organic linkers create nanoscale pores in the structure. The attractive properties of MOFs come from this stable, tunable, and uniform porous network. Tunability is a unique function of MOFs compared to other crystalline structures such as zeolites and carbons. Controlling the porous network parameters allow the control of MOFs properties. The most common pore shapes in MOF structures can be listed as cubic, triangular, and hexagonal. However, the most important pore property is the pore size rather than the pore shape. Porous MOF structures can be divided into two categories according to their pore size; microporous MOFs (pore size <2 nm) and mesoporous MOFs (2-50 nm). Most of the MOF structures investigated are microporous MOF structures. However, mesoporous MOFs have recently attracted attention. Porous units of MOF can assemble to form cage-type, channel-type, and chiral MOF structures. The size and shape of the pores are changed by using different types of ligands or metal centers to obtain different functionalities. For example, smaller pore sizes are favorable for gas storage applications while relatively larger pore sizes are preferred for catalytic applications.
Metal organic frameworks are grouped together based on various different features such as their discovery places, same symmetry, etc. The most known MOF groups are HKUST, UiO, MIL, LIC, and IRMOF. These groups are branched over time. One of the well known MOF structures is HKUST-1. It is also widely known as Cu-BTC (copper benzene-1,3,5-tricarboxylate). This material sets an example for the common properties of MOF structures such as high surface area, pore-volume, tenability, and adsorption capacity. However, there is a plateau of different properties MOFs can offer such as mechanical, optical, and magnetic properties that can be induced through different synthesis methods and different building methods.
Synthesis of MOFs
Synthesis of MOFs requires two basic components and most of the time solvent as a reaction medium. The basic components of MOFs are inorganic SBUs and organic linkers. Inorganic SBUs can be transitional metals or rare earth materials. The commonly used metal precursors are divalent atoms such as Zn2+, Cu2+, Fe2+, Ni2+, Co2+, Cd2+, Pb2+, Ru2+, Zr2+ or trivalent oxidation state such as Fe3+, Cr3+, Al3+. In addition, Li, B, and Ca ions and lanthanide series which contain La, Ce, Pr, Nd, Pm, Sm, Eu, Gd, Tb, Dy, Ho, Er, Tm, Yb have attracted attention. Li, B, and Ca ions attract attention due to their lightweight while lathanide series attract attention due to their excellent coordination properties and unique chemical characteristics. Inorganic SBUs act as a directional point during MOF synthesis since organic linkers cluster around them.
Organic linkers are ions or neutral molecules that surround and bond with metal centers. These molecules act as Lewis bases. They generally contain carboxylates, or anions, such as phosphonate, sulfonate, and heterocyclic compounds.The wide variety of available linker molecules enables the construction of MOFs with various unique properties. The solvent medium is very important for the synthesis of desired MOF structures since it affects the coordination behavior of the metal centers. Solvents can be water and organic molecules such as ethanol, methanol, dimethyl sulfoxide (DMSO), etc. Solvents have three different roles in the synthesis process; solvent for organic linkers and metal clusters, structure-directing agent, and ligand to coordinate the metal ions.
The most commonly used synthesis method for MOF synthesis is the solvent evaporation method. Other synthesis methods are diffusion method, hydro(solvo)thermal method, microwave reaction method, electrochemical method, mechanochemical method, and ultrasonic method.
The solvent evaporation method provides crystal growth in saturated solutions. The remaining solvent is evaporated gradually through the following days after the completion of the reaction. The rate of evaporation is an important parameter in this method and allows the manipulation of crystal properties.
The diffusion method can utilize solvent interfaces as well as physical barriers to control the diffusion rate of reactants and products. In the solvent liquid diffusion method, two liquid layers of different densities are observed. One of these layers contains the precipitant solvent while the other is the product solvent. Crystallization occurs at the interface of these two liquid layers after the gradual diffusion of precipitant solvent. When physical boundaries are present the diffusion rate can be controlled much more efficiently and allow the formation of single crystals. These physical boundaries can be two vials with different sizes or gels.
The hydro(solvo)thermal methodis a wet chemical synthesis method that was originally used for the synthesis of zeolites. This method then adapted to the synthesis of MOFs. In this method, solvent precursors are used for the assembly of the product. The precursor mixture is left to complete the reaction within a sealed container in an autoclave at 80-260°C. The cooling rate and the pH are the important parameters affecting the quality of the end product.
The microwave method is a novel approach to MOF synthesis. The microwave method does not directly produce crystals rather creates nucleation sites by creating small metal and oxide particles through sudden temperature rise. This method is valuable because it has the potential to reduce the duration of synthesis. Nucleation sites created by this method can be further used for crystallization through either solvent evaporation or hydro(solvo)thermal method.
The electrochemical method promotes the crystallization of MOFs by the application of a current to the solution. Electrochemical reactions take place in an electrochemical cell at relatively high speeds. The electrochemical method is preferred for the MOF powder synthesis on the industrial scale. The advantages of this method are avoiding anions such as nitrates from metal salts, lower reaction temperatures, and quick synthesis compared to other widespread methods. Furthermore, this method allows the tuning of MOF properties by simply changing the voltage or imposing pulses on the reaction medium.
The mechanochemical method uses mechanical force for the chemical reaction. Intermolecular forces are broken by mechanical force to promote the chemical reaction. The most important feature of this method is that it doesn’t require a solvent medium. Avoiding organic solvents is important for environmental concerns and through mechanochemical methods reactions can take place under ambient conditions in the absence of a solvent. A high quantity of products can be achieved in a matter of minutes through this method. Furthermore, low solubility metal oxides can be utilized for MOF production as opposed to the solvent containing methods broadening the variety of SBUs. At the same time, it is possible to use a small amount of solvent in order to increase the mobility of reactants on the molecule scale. This method is called liquid assisted grinding (LAG) and the presence of the solvent is found to accelerate the chemical reaction.
The ultrasonic method applies high-energy ultrasound on the reaction mixture. Ultrasound is a cyclic mechanical vibration with the frequencies higher than the human hearing. Ultrasound application promotes the dissolution of reactants. This method allows the environmental friendly, quick, and energy-efficient production of MOFs under ambient conditions.
Application Areas
The versatility of MOFs provides a wide variety of application areas. Not only pure MOF structures have different potential application areas but also the composite MOF structures enrich these possibilities. Various different materials such as polymers, active species, metal nanoparticles, quantum dots, carbon nanotubes, graphene, and enzymes are used to form composites with MOFs. Practical applications utilize the high surface area, porous structure, thermal conductivity, electrical conductivity, and surface activity of MOF structures. The application areas of MOFs can be listed as;
- Gas storage
- Gas adsorption
- Gas separation
- Catalysis
- Sensors
- Energy storage
- Proton Conductivity
- Biomedicine
Click Image to Learn About Nanobiotechnology
Conclusion
Metal organic frameworks are novel porous crystalline materials that have attracted attention in the last decades. They combine the unique properties of nanoscale structures and porous networks and provide high surface areas, high pore volumes, adsorption, and catalytic properties. The importance of MOFs stems from their tenability and distinguishes them from formerly known crystalline structures such as zeolites and carbon nanostructures. These exceptional properties have the potential to be utilized in gas adsorption, storage and separation, heterogeneous catalysis, biomedical applications, and energy storage. Various researches and practical applications have proven MOFs significant role in today's scientific world as well as the future of science
References
1.Olajire, A. A. (2018). Synthesis chemistry of metal-organic frameworks for CO2 capture and conversion for sustainable energy future. Renewable and Sustainable Energy Reviews, 92, 570-607.
2.Safaei, M., Foroughi, M. M., Ebrahimpoor, N., Jahani, S., Omidi, A., & Khatami, M. (2019). A review on metal-organic frameworks: synthesis and applications. TrAC Trends in Analytical Chemistry.
3.Qiu, S., & Zhu, G. (2009). Molecular engineering for synthesizing novel structures of metal–organic frameworks with multifunctional properties. Coordination Chemistry Reviews, 253(23-24), 2891-2911.
4.Xuan, W., Zhu, C., Liu, Y., & Cui, Y. (2012). Mesoporous metal–organic framework materials. Chemical Society Reviews, 41(5), 1677-1695.
Recent Posts
-
Nanocomposites in Food Packaging
The utilization of nanocomposites in food packaging represents a significant advancement in the fiel …19th Apr 2024 -
What is the Difference Between 7075 and 6061 Aluminum Alloy?
When comparing 7075 aluminum alloy to 6061 aluminum alloy, it's essential to understand their disti …5th Apr 2024 -
Iron-Air Batteries: The Ultimate Guide
Iron-air batteries represent a significant breakthrough in energy storage technology, offering a sus …29th Mar 2024