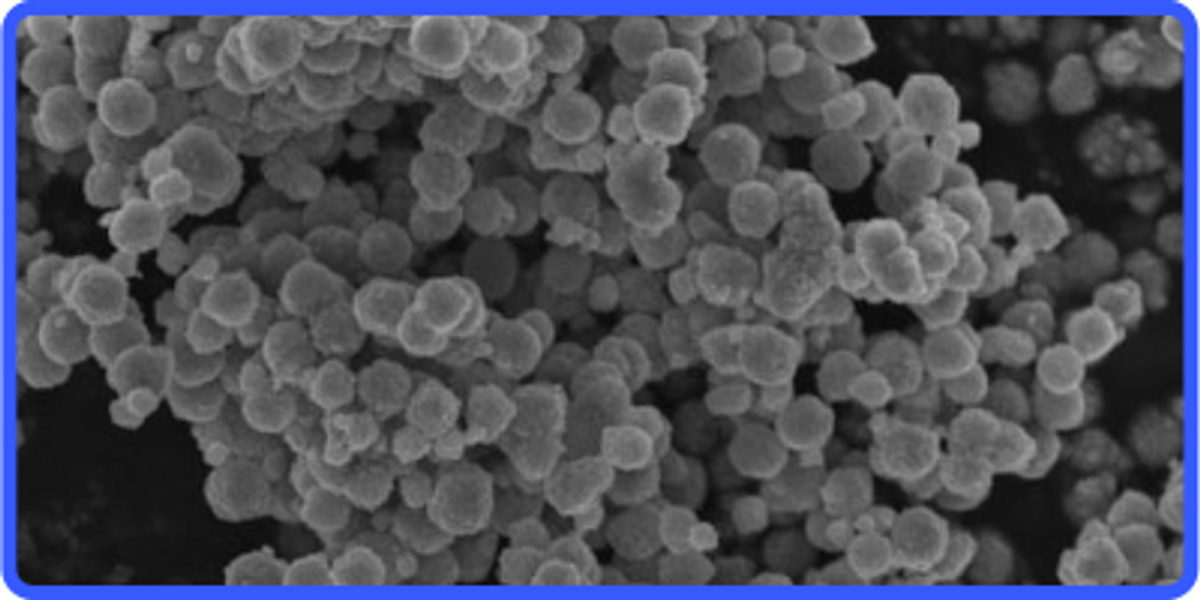
Rutile and Anatase TiO2 Nanoparticles and Applications
Titanium Oxide (TiO2) is an important material having a lot of vital properties and applications such as the production of titanium metal, titanium oxide nanoparticles, etc. It has two important types which are rutile titanium dioxide and anatase titanium dioxide. The key difference among them is in their appearance. Anatase Titanium Dioxide is colorless, whereas Rutile Titanium Dioxide is usually found in dark red appearance. Rutile titanium dioxide is optically positive, whereas Anatase titanium dioxide is optically negative. This articles discusses the main differences between the two types of Titanium Dioxide and also describes their applications.
Rutile Titanium Dioxide Nanoparticles
As already mentioned, Rutile Titanium Dioxide appears in dark red color and it has high stability. Moreover, it is the most commonly found type of Titanium Dioxide and is usually found in metamorphic and igneous rocks, which are subjected to high-pressure and high-temperature conditions.
While talking about the rutile’s crystalline structure, it has a tetragonal unit cell having oxygen anions and titanium cations. The coordination number of titanium cations (Ti+4) is 6, while the coordination number of oxygen anion (O2-) is 3. Some vital properties of rutile titanium dioxide nanoparticles are given below:
- Greater dispersion
- Higher birefringence
- Greater refractive index (RI) at visible wavelengths
It has a lot of useful applications, the main ones are the manufacturing of metallic titanium and titanium dioxide pigments. Moreover, in fine form, it is used for manufacturing plastics, papers, and paints. The finely pulverized form of titanium dioxide is white in color. Furthermore, the nanoparticles of rutile titanium dioxide have the ability to absorb UV rays and are transparent to visible light. That is the reason that they are used in the manufacturing of cosmetics.
Anatase Titanium Dioxide Nanoparticles
The nanoparticles of Anatase titanium dioxide are found in colorless or yellow color. The natural form of Anatase titanium dioxide contains a lot of impurities which makes it dark.
The crystalline structure of Anatase titanium dioxide is tetragonal and are different from rutile titanium dioxide. The appearance of Anatase titanium dioxide is metallic. It can also be synthesized to be used in the manufacturing of semiconductors.
9 Differences between Rutile and Anatase Titanium Dioxide Nanoparticles
The 9 main differences between the two types of Titanium Dioxide are given below:
1.Appearances of Rutile and Anatase TiO2 Nanoparticles
Rutile titanium dioxide is a component that contains mainly TiO2 with a dark red appearance, whereas Anatase titanium dioxide has colorless to blue appearance.
2.Colors of Rutile and Anatase TiO2 Nanoparticles
Rutile titanium dioxide has dark red color and the finely pulverized rutile titanium dioxide has a bright white color, whereas Anatase titanium dioxide has a dark color in the presence of impurities, but its pure form is colorless or white.
3.Optical activity of Anatase and Rutile Titanium Dioxide Nanoparticles
Rutile titanium dioxide is optically positive, whereas Anatase titanium dioxide is optically negative.
4.Occurrences of Anatase and Rutile TiO2 Nanoparticles
Rutile is a type of titanium dioxide that is found naturally in huge quantity as compared to anatase titanium dioxide.
5.UV absorptions of Anatase and Rutile Titanium Oxide Nanoparticles
Ultraviolent absorption by rutile titanium dioxide is great, whereas ultraviolet absorption by anatase titanium dioxide is low.
6.Hardness of Rutile and Anatase Titanium Dioxide Nanoparticles
The hardness of Rutile titanium dioxide is great, whereas Anatase titanium dioxide is not that much hard.
7.Specific gravity of two types of TiO2 Nanoparticles
The density of Rutile titanium dioxide is high, whereas the density of the anatase titanium dioxide is low.
8.Different crystal structures of two types TiO2 Nanoparticles
The anatase and rutile titanium oxide crystal structures consist of interconnected TiO2 octahedra. The difference between the two is that the degree of distortion of the octahedron is different for both.
9.Price Differences between Anatase and Rutile TiO2 Nanoparticles
Due to the relatively high price of rutile titanium dioxide, it is often used in high-end products. Rutile titanium dioxide is not only used in the production of high-quality water-based printing materials such as paints, inks and plastic woodwork. On the other hand, Anatase titanium dioxide is less expensive than rutile titanium dioxide and is mainly involved in the production of plastics, printing inks, printing inks, and coatings.
Since the price of rutile titanium dioxide is relatively high, there are many counterfeit products on the market, including the following ones:
(a) Packaging of domestic titanium dioxide on imported titanium dioxide.
(b) Impregnation of the rutile with anatase titanium dioxide or incorporating a portion of anatase titanium dioxide in the rutile type.
(c) Mixing filling materials such as calcium carbonate, lithopone and barium sulfate in rutile type titanium dioxide or anatase titanium dioxide.
The differences between the main chemical and physical properties of Rutile and Anatase Titanium Dioxide are shown in the table below:
| Sr. No | Rutile Titanium Dioxide | Anatase Titanium Dioxide |
| 1 | Square crystal system | Crystal cone shape |
| 2 | Relative density (8 - 3.9) | Relative density (4.2 – 4.3) |
| 3 | Refractive index (2.52) | Refractive index (2.71) |
| 4 | Mo hardness 5.5 - 6 | Mo hardness 6 – 7 |
Production methods of Rutile and Anatase titanium Dioxide Nanoparticles
Titanium Oxide can be obtained through two processes: the sulfate process and the chloride process, which use the two main minerals, ilmenite and rutile, they are particularly abundant in Australia and South Africa.
1)Sulfate Process in the production of TiO2 Nanoparticles
The sulfate process involves three phases:
1) Mineral Dissolution
2) Hydrate Titanium dioxide formation
3) Anhydrous titanium dioxide formation
The mineral used in this process is the ilmenite FeTiO3. After grinding, it is treated with sulfuric acid with the formation of a mixture of sulfates, titanyl sulfate, iron (II) sulfate and iron sulfate (III).
FeTiO3 (s) + 2H2SO4 (aq) → TiOSO4 (aq) + FeSO4 (aq) + 2H2O (l)
Before extraction of titanium dioxide, the ferric ion is converted to a ferrous ion using iron filings:
2Fe3+ (aq) + Fe (s) → 3Fe2+ (aq)
Any solids present are removed by filtration after sedimentation. The solution is cooled and the iron (II) sulphate crystals precipitate which, in turn, are removed by filtration.
The residual solution consists of titanyl sulfate so the next step involves the production of hydrated titanium dioxide according to the reaction:
TiOSO4 (aq) + H2O (l) → TiO2 (s) + H2SO4 (aq)
The hydrated form of titanium dioxide is brought to a temperature of about 800 °C to which it loses its hydration water.
2)Chloride Process in the production of Titanium Dioxide Nanoparticles
The chloride process involves the transformation of rutile to titanium chloride (IV) and the subsequent oxidation of the latter. Rutile is heated to 900 °C in the presence of chlorine and coke with the formation of titanium chloride (IV) which is a volatile compound. The latter is heated in the presence of oxygen at 1200 °C.
TiCl4 (g) + O2 (g) → TiO2 (s) + 2Cl2 (g)
Applications of Titanium Dioxide Nanoparticles
Titanium dioxide not only provides hiding power but also acts as an ultraviolet shielding agent. Titanium dioxide is divided into two types: anatase and rutile. Anatase titanium dioxide and rutile titanium dioxide have different structures and have different applications. For example, Anatase titanium dioxide is used for interior wall cladding because it is easy to pulverize and rutile is often used for exterior wall cladding due to its aging resistance. Given below are the main applications of the two types of Titanium Dioxide.
8 Uses of Anatase Titanium Dioxide Nanoparticles
Anatase Titanium dioxide is extensively used in plastics, ink, coatings, paper, rubber, chemical fiber, chemical, ceramics, pharmaceutical, food, and some further industries. The main uses of Anatase titanium dioxide are stated below:
1. Anatase titanium dioxide has a good photocatalytic effect and is widely used in photocatalysts and air purifiers.
2. Anatase titanium dioxide due to the huge specific surface has been broadly used in solar cells, environmental purification, photocatalysis, catalyst media, gas sensors, and lithium batteries.
3. Anatase titanium dioxide is used in papermaking, it can play the role of fluorescence and brightening and increase the whiteness of the paper. In the field of printing, the thicknesses of coatings of less than 100 millimeters are required, which is why very fine titanium dioxide pigments are used. Fillers such as chalk, talc or kaolin are used for the paper industry. Titanium dioxide pigments are used for making a very white paper and should be opaque and very thin. It is also applied as a coating to make "artistic" paper.
4. It is also used in the manufacturing of ink. It is an indispensable white pigment in advanced inks.
5. Anatase Titanium dioxide has also applications in the textile and chemical fiber industry. They remove the greasy appearance on fibers caused by the translucent properties of the resin.
6. Anatase Titanium dioxide is used as a dye in the rubber industry and it also has reinforcing, anti-aging and filling effects.
7. Other areas of application of Anatase titanium dioxide include the ceramic industry, the manufacturing of white cement and the coloring of rubber or linoleum.
8. In addition to being a pigment, titanium dioxide is used as a catalyst, as a protector of the skin against UV rays in some cosmetic creams. In nanoparticle form, it is used in sunscreens, food dyes, self-cleaning surfaces, and dyes.
7 Uses of Rutile Titanium Dioxide Nanoparticles
Its applications cover all industries as we can see in the following examples:
1. RutileTitanium dioxide is non-toxic, with strong photocatalytic action and excellent transparency, therefore, it is used in coatings and products for air purification in indoor environments.
2. RutileTitanium dioxide has antibacterial functions due to their photocatalytic property which can abolish bacteria or overpower their reproduction.
3. RutileTitanium dioxide also improves the mechanical properties of particles through dispersion.
4. RutileTitanium dioxide can be used in various military fields.
5.Titanium dioxide pigments are also employed as ultraviolet absorbers in products for, cosmetic powders, soaps, tanning, cigarette paper, creams, toothpaste, and the cosmetic industry.
6. Rutile Titanium dioxide is used universally in the paints and coatings industry, and it has replaced any other white pigment in the market.
7. Rutile Titanium dioxide is also used to color plastic items such as toys, electronics, cars, furniture, packaging, and so on. The pigment of titanium dioxide absorbs part of the UV radiation protecting its content.
Conclusion
Rutile and anatase Titanium Dioxide nanoparticles are 2 mineralogical types of titanium dioxide. The key difference among the two is their appearance as Rutile Titanium Dioxide is usually found in dark red appearance, whereas anatase titanium dioxide is colorless. They have very important applications in different industries, particularly in the paint industry.
Recent Posts
-
What is the Difference Between 7075 and 6061 Aluminum Alloy?
When comparing 7075 aluminum alloy to 6061 aluminum alloy, it's essential to understand their disti …5th Apr 2024 -
Iron-Air Batteries: The Ultimate Guide
Iron-air batteries represent a significant breakthrough in energy storage technology, offering a sus …29th Mar 2024 -
Discovering the Power of 2D Materials
In material science, the discovery of two-dimensional (2D) materials represents a transformative de …22nd Mar 2024