Holey Graphene: A Promising Graphene Derivative
Holey graphene, also referred to as perforated graphene, is created by creating in-plane holes in the basal planes of materials containing graphene.
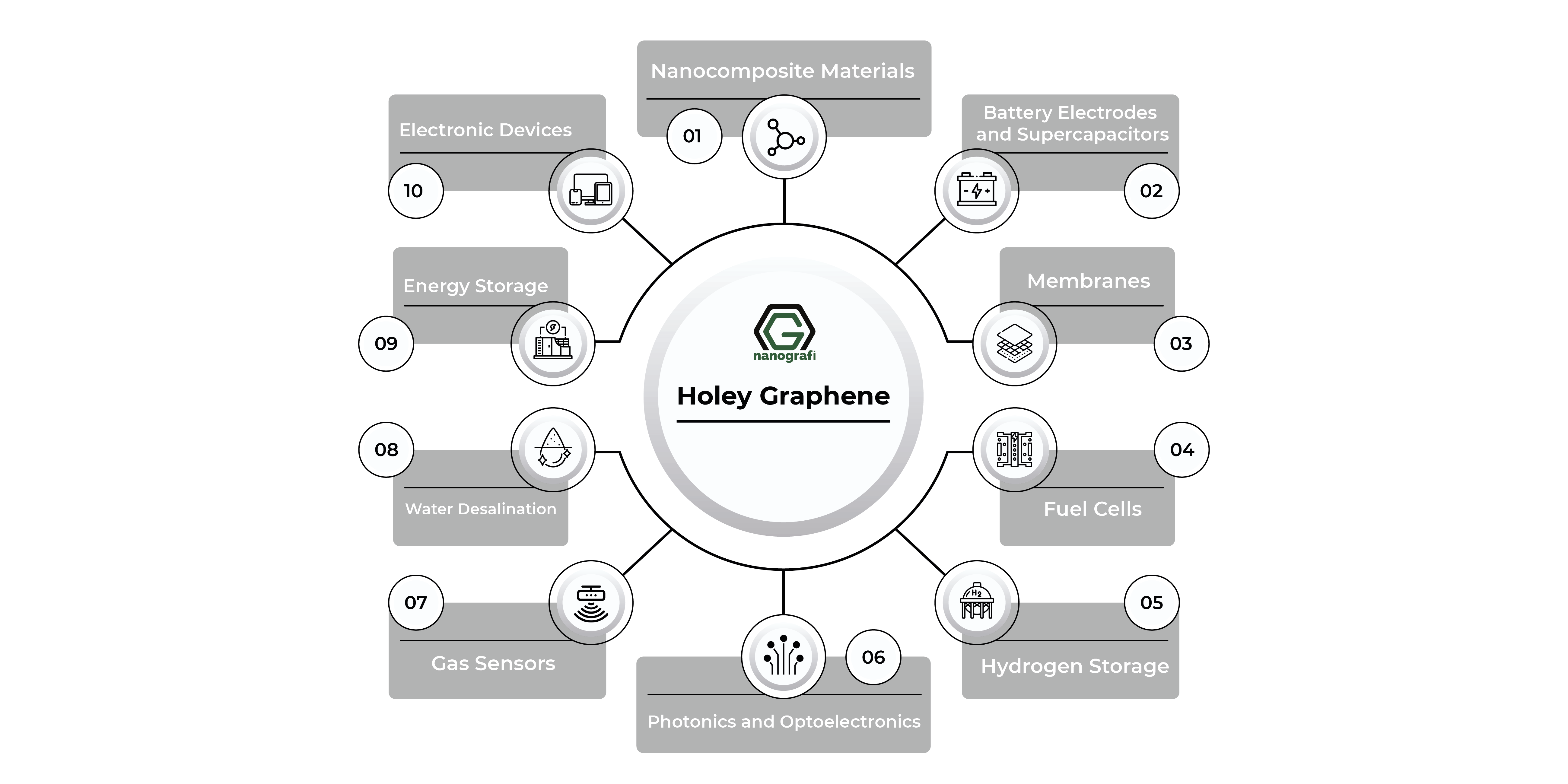
Holey graphene-based materials have gained a lot of interest because of their extraordinary qualities, such as high electrical conductivity and high surface area, which are achieved by combining the advantages of holes with graphene. With the help of these exceptional qualities, holey graphene-based materials have outperformed graphene in its purest form and shown their versatility in a variety of applications, such as electrical energy storage (supercapacitors and Li-ion, Li-air, Li-S, and Na-ion batteries), energy conversion (electro-water splitting and dye-sensitized solar cells), water desalination, bioseparation, fuel cells, gas sensors, hydrogen storage, and dye degradation systems.
Therefore, it is crucial to identify the key characteristics of holey graphene-based materials and their possible industrial applications. The most recent developments in cutting-edge hole generation techniques for materials based on graphene are addressed, along with their operating principles, related problems, and potential solutions. The article aims to provide fresh ideas for the development of holey graphene-based materials as a formidable candidate for many applications, as well as critical perspectives on the state of the art and future possibilities.
Nanografi provides SuperGraphene (also known as Holey Graphene) products & solutions at every level.
Introduction
Graphene's structural descendant, holey graphene (hG), is also known as graphene nanomesh. To create holes dispersed across and through the atomic thickness of the graphene sheets, a significant number of atoms from the graphitic plane must be removed. These holes, which occasionally have several functional groups at their edges, impart qualities that are unusual for intact graphene yet helpful for a variety of applications. The methods for preparing hG and the associated applications that make use of the distinctive structural theme of these materials are covered in this article. The future of this new class of graphene derivatives is then discussed.
What is the Holey Graphene?
A brand-new class of in-plane porous graphene derivatives is called holey graphene. In several applications, including nanoelectronics, sensors, energy storage, and membranes, it performs better than intact graphene. A flawless two-dimensional graphitic crystal with just one layer of carbon atoms makes up an ideal graphene sheet. It has been used in electronics, sensors, composites, and energy storage since graphene's first isolation in 2004 because of its high surface area, high electrical and thermal conductivity, excellent mechanical strength, thermal and chemical stability, and ability to be modified to introduce various chemical functionalities.
Synthesis of Holey Graphene
Numerous techniques have been developed in the last ten years to produce monolayer or few-layered graphene sheets. The "Scotch tape" method of mechanical exfoliation, which produces huge area sheets with very low defect densities, produces the highest-grade sheets. Because it only produces a small number of sheets on supported substrates, this approach is not scalable. The process of oxidizing and exfoliating graphite to produce graphene oxide (GO), which may then be reduced to return it to its graphitic structure, is the most scalable one for applications like composites and energy storage. The resulting graphene, known as reduced graphene oxide (rGO), has imperfections but is readily manufactured in laboratories in grammes and has recently been industrialized to tonnes of quantities.
Other techniques, including chemical vapour deposition (CVD) growth and liquid exfoliation of graphite, have also been industrialized and have given graphene materials with qualities and quantities between the aforementioned two extremes as well as specific property combinations needed for some applications. While physically exfoliated graphene sheets often have great atomic order, graphene sheets made using several other processes invariably have flaws that range in size from an atom to a nanometer. Defects on graphene sheets would appear to be undesirable at first appearance because they would negatively affect the material's mechanical strength and electrical conductivity. However, structural flaws at the nanoscale give unique physical and chemical properties to nanosized materials as a result of quantum confinement processes, providing qualities that are desired for particular applications. Defects, for instance, may make it easier for functional groups to attach, which may affect processing characteristics or interactions with other materials like polymer or biological agents, making these functionalized graphene materials more useful in biological and polymeric composite applications.
The topic of this article is "holey graphene," a distinct class of faulty graphene known variously as "graphene meshes" or "graphene nano meshes." To provide considerable benefits for a variety of applications, hG purposely introduces nanometer-sized or even bigger ('mesoscale') holes. For instance, the formation of many holes can lead to the remaining graphitic structure being quantum confined, which has a substantial impact on the electrical characteristics of the nanosheets. The many hole-edge functional groups offer many more active sites for sensing applications and can boost sensitivity or reactivity to specific chemical or biological species. The existence of holes may facilitate cross-plane ion movement in energy storage applications, enabling high charge/discharge rate devices. A discussion of many illustrative applications that are made possible by the structural motifs of hG follows a methodical presentation of various hG preparation techniques.
High-fidelity and Precise Approaches for Holey Graphene (hG)
Early research on holey graphene (hG) primarily focused on the development of high-precision and high-fidelity techniques for creating voids within pristine graphene sheets. This research was primarily driven by the need to address two key questions:
- Can substantial faults or voids, ranging from nanometer to larger scales, be introduced into a graphene sheet while preserving its mechanical integrity?
- How does the alteration of the conjugated structure affect the electronic properties of graphene when quantum confinement is induced?
To explore these questions, scientists employed a range of techniques, encompassing both low-throughput methods like nanoscopic electron beam drilling and high-throughput methods such as nanolithography or templated growth. High-throughput techniques enabled the fabrication of meticulously organized arrays of voids with precisely controlled shapes on graphene surfaces. While these precision techniques are primarily applicable to substrate-supported graphene, they have paved the way for materials suitable for morphological investigations, electronic testing, and various applications.
Notably, highly ordered arrays of voids in hG are often likened to interconnected graphene nanoribbons. Graphene nanoribbons, distinguished by their high electron mobility compared to pristine graphene sheets and possession of a finite band gap, make them ideal candidates for graphene-based field-effect transistor (FET) devices. However, manufacturing high-quality, narrow-width graphene nanoribbons on a large scale has proven challenging. The high-fidelity hG nanoribbon mimics have demonstrated promising performance and have introduced innovative possibilities for FET applications.
Bombardment with Ions or Electrons
The utilization of mechanical force is an obvious option in the macroscopic world to create holes through a thin layer of material. High-energy bombardment with electrons or ions was one method for producing holes through graphene sheets. For instance, the drilling of nanometer-sized holes in graphene sheets using an electron beam in a transmission electron microscope (TEM) was observed in real-time imaging. In one of the earliest attempts, Fischbein and Drndic suspended multilayer graphene sheets that had been mechanically exfoliated over a hole in a SiNx membrane and condensed the imaging electron beam to the nanometer range. Because of the electron beam's high energy, it was possible to vaporize through the graphene layers and create pores with a circular shape. Pores of various forms (circular or line-shaped) and arrays of pores were generated at a rate of a few seconds per hole by regulating the position and/or movement of the electron beam. The threshold electron beam energy required for hole ablation in defect-free graphene was around 80 keV, and the carbon displacement cross-section increased fast with energy. However, the ablation energy barrier was dramatically lowered by imperfections, doped atoms, and contaminations.
Nanolithographic Methods
Compared to electron beam drilling, nanolithographic etching is a high-throughput method to produce hG with periodic arrays of holes and narrow hole-size distributions. A typical nanolithographic etching process consists of several common steps:
- Graphene sheets are deposited or synthesized on a substrate
- A porous template or mask is prepared to cover the top surface of the graphene sheets
- An etching process is conducted
The Template or Mask is Removed to Yield hG Products
Block copolymers, polystyrene (PS) nanospheres, anodic aluminum oxide (AAO), ad patterned photoresist layers were some of the several materials used to make the porous templates or masks. These templates must not react negatively with graphene and must only add morphologically to the etching process.
Block copolymer lithography, a multi-step nanolithographic process employing a block copolymer as the template, was initially separately described in 2010 by Bai et al. and Kim et al., who created hG ('graphene nano meshes') with highly ordered mesh-like structures.
Templated Expansion
Recent years have seen a significant amount of research towards the bottom-up growth of graphene using CVD techniques using gaseous carbon precursors. In a typical CVD growth process, a gaseous carbon feedstock, such as methane, is introduced into a heated furnace and decomposes on the surface of a supporting smooth metallic substrate that has an extremely low carbon solubility (typically Cu or Ni). This enables the two-dimensional growth of graphene crystals. Graphene is first generated or deposited onto a substrate coated with the nano templates in the nanolithography methods for hG outlined in the preceding section, and then it is selectively erased. The nano templates are first deposited onto an active substrate in the templated growth of hG, after which graphene is grown by CVD.
Scalable Techniques
It takes a lot of active materials to make many non-electronic applications like composites and energy storage possible. For such reasons, the high-precision hG synthesis techniques covered in the previous section are typically insufficient. Getting enough graphene starting material is the first stage in producing large amounts of hG. As was said in the Introduction, one of the easiest and most scalable ways to make graphene is by using GO nanosheets, which are made by oxidizing and exfoliating graphite in the liquid phase. Most of the scalable hG preparation techniques described thus far began with GO or its reduced version (rGO). The predominant seeding sites for the hole expansion to generate hG are typically the numerous defect sites in GO or rGO from the highly oxidative synthesis conditions. It should be emphasized that the majority of scalable techniques for converting GO into hG can also be employed to create substrate-supported graphene sheets, however, the conditions must be changed to prevent excessive oxidation.
Oxidation in the Liquid Phase
In hG synthesis, liquid-phase oxidation techniques utilizing traditional wet chemistry are frequently used. In many instances, holey graphene oxide (hGO) nanosheets, the highly oxidized and water-soluble form of hG, were the direct outcomes of liquid-phase oxidation techniques. hGO can be lowered using GO-compatible reduction techniques to more electrically conductive hG. The comparison between hGO and hG is very similar to that between GO and rGO.
Etching in the Gaseous Phase
As-prepared graphene sheets, particularly those made from thermally reduced GO (t-rGO), which was produced by rapidly heating GO to achieve simultaneous reduction and exfoliation, have a lot of inherent point defects. These point defects may develop from the parent GO due to the circumstances for oxidative synthesis or may develop after rapid heating as a result of the prompt release of gaseous byproducts like CO2. Our team and other teams have recently expanded on this component of the thermal exfoliation technique to create hG via controlled partial air oxidation. The easiest ways to produce hG using GO or rGO as the starting material were these processes since they were normally carried out in the air without the use of solvents or other chemical agents.
Activated carbon (AC) is a substance with a high surface area that has been industrialized and is frequently used as an electrode and adsorbent. The chemical treatment of a carbon stock with certain corrosive chemicals, such as potassium hydroxide (KOH), followed by carbonization is a typical process for producing AC. Similar techniques were utilized by Ruoff and colleagues to treat microwave-exfoliated GO (MEGO), a kind of reduced GO produced by a quick microwave heating method, and create the high-porosity activated graphene material known as "a-MEGO."
Applications for Holey Graphene
Target applications for hGs produced using high-precision techniques like nanolithography and templated growth are mostly found in nanoelectronics, such as FETs and sensing devices based on FETs. Although these programs were only briefly covered in the prior parts, other reviews have greater information. Several more significant uses of hGs' structural motifs, particularly those from scalable preparation techniques, are mentioned in this section. While all of these applications benefit from using pristine graphene, the performance of hGs is improved by the presence of holes, hole-edge functional groups, or both.
Supercapacitor and Battery Electrodes
Due to their superior electrical conductivity and large surface area, carbon nanomaterials, particularly graphene, are appealing candidates for use as improved electrode materials in energy storage systems like supercapacitors and batteries. However, restacking graphene sheets in their solid state drastically reduces the surface area that the electrolyte ions may access, resulting in a device performance that is below that predicted by theory. The introduction of "pores" between the nanosheets to improve ion access is a typical technique to increase the accessible surface area of graphene. For use in energy storage applications, solid-state graphene having this topology is known as "porous graphene". The notion of hG is essentially distinct, even though the holes on hG sheets can be thought of as two-dimensional pores.
To find out more, you can read A novel approach to improve graphene-based supercapacitors.
Catalysis and Energy Storage with N-doped hG
The oxygen-containing functional groups connected to the hole edges of conventional hG materials showed some catalytic activity, but further work is required before they can be used in real applications. Recently, expensive noble metal catalytic systems have found good metal-free substitutes in graphene hetero-atomically doped with elements like N (the most prevalent), B, P, or S. N-doped graphene has more electronegativity around the doping sites than undoped graphene, which increases its catalytic activity for reactions like the oxygen reduction reaction (ORR) and oxygen evolution reaction (OER). One of the current focused research and development topics is ORR and OER, both of which are crucial in oxygen-related energy storage systems such as fuel cells and metal-air batteries.
Membranes
An illustration of hG is single-layer graphene with in-plane pores, which has been suggested for use in a variety of membrane applications, including gas separation, water purification, and biosensing. Both the preliminary experimental findings and theoretical projections are encouraging. For instance, the initial investigations on drilling electron beam holes in graphene sheets had the direct application of using the resulting nanopore to monitor the translocation of biomolecules like DNA. It was possible to observe and study the disturbance (i.e., blockage) in the solution ionic current as these big molecules gradually moved through the hole. Because graphene sheets are so thin, it may be possible to sequence DNA at high resolution.
Holey or porous graphene is a structural variant of graphene that has drawn a lot of interest because of its special characteristics and prospective uses in many fields of science and technology. The synthesis processes of porous or holey graphene/graphene oxide are thoroughly summarized in this article, along with their prospective applications in several fields.
Electrochemical Energy Storage with Holey Graphene
Due to their intriguing physicochemical features, graphene and its hybrids have been regarded as attractive options for electrochemical energy storage. They have unsatisfactory areal or volumetric energy densities, though, and their rate performance is often subpar. Due to the agglomeration and restacking of graphene nanosheets during electrode assembly, these shortcomings result in a small accessible surface area and poor ion diffusion capability. Perforation on the graphene planes is used to create porous structures in the graphene-based nanomaterials, which addresses the aforementioned problems. Ion transport can be accelerated over the graphene sheets and eventually reach the inner electrode surface thanks in part to in-plane holes.
Functionalized Holey Graphene
The oxygen reduction reaction has been employed with amine-functionalized holey graphene (AFHG), a material created via the hydrothermal reaction of GO with ammonia and the subsequent KOH etching (ORR). Since AFHG has a greater current density and more positive half-wave and onset potentials for the ORR than graphene, nitrogen-doped graphene (NG), and amine-functionalized graphene (AFG), it is clear that it is extremely active for the ORR and has higher electrocatalytic activity than these materials. The commercial JM Pt/C 40 wt% is more kinetically difficult to work with than AFHG, even though it also shows a somewhat larger overpotential towards ORR. The presence of the electron-donating group (such as amine) and numerous holes in its sheet plate and porous structure in its randomly stacked solid, provide AFHG with higher electrical conductivity, more active edge N atoms, and easier access to oxygen, respectively, could be attributed to its higher electrochemical performance. According to the stability experiments, AFHG is more stable than graphene, NG, AFG, and the JM Pt/C 40 wt%. It also shows greater resistance to methanol crossover and CO poisoning. During the first 10 hours of the ORR, AFHG loses just 7% of its initial activity, and adding methanol or CO has little impact on its oxygen reduction activity.
Advantages of Holey Graphene (Holes)
HG-based nanomaterials may offer several structural advantages for EES applications in contrast to graphene-based nanosheets. In addition to changing the geometry of the graphene nanosheets, the holes also lessen the van der Waals force that holds the sheets together. As a result, the following benefits of HG-based nanomaterials for EES can be drawn:
The electrochemical processes can be promoted by HG-based materials with high surface areas and plenty of active sites, considerably raising the specific energy density.
Even in a severely compressed state, the pores made in the graphene sheets offer enough cross-plane pathways for ionic diffusion, leading to favourable ion kinetics and increased volumetric energy density.
To accommodate the volume changes brought on by electrochemical reactions and provide outstanding cycle performances, nanomaterials' holes are advantageous.
Restacking of graphene sheets can efficiently reduce distortions caused by holes that have formed. To increase the penetration of electrolyte ions into the electrode surface and to promote quick charge transfer, respectively, more horizontal ion channels and electron transfer junctions between sheets can be made.
In comparison to graphene-based materials, HG-based materials are better at transporting charges thanks to nanoholes, allowing for the creation of thick electrodes with high volumetric energy/power densities.
Conclusion
High mechanical strength, vast surface area, high electrical and thermal conductivity, high thermal and chemical stability, and varied chemistry were all retained by hG despite the existence of arrays of holes that penetrated the atomic thicknesses of the two-dimensional nanosheets. Additionally, the distinct structural motif of this new class of graphene derivatives makes it significantly more beneficial than its parent counterpart in a variety of applications by enabling straightforward through-plane mass transport, straightforward functionalization at or near the hole edges, and altered electronic properties.
To discover the latest articles in graphene derivatives, you can visit Blografi.
References
A Novel Approach to Improve Graphene-based Supercapacitors - Nanografi Nano Technology. (n.d.). Retrieved April 19, 2024, from https://nanografi.com/blog/a-novel-approach-to-improve-graphenebased-supercapacitors/
Chemical Vapor Deposition CVD Graphene - Nanografi Nano Technology. (n.d.-a). Retrieved April 19, 2024, from https://nanografi.com/blog/chemical-vapor-deposition-cvd-graphene/
Chemical Vapor Deposition CVD Graphene - Nanografi Nano Technology. (n.d.-b). Retrieved April 19, 2024, from https://nanografi.com/blog/chemical-vapor-deposition-cvd-graphene/
Discovery of graphene - Graphene - The University of Manchester. (n.d.). Retrieved April 19, 2024, from https://www.graphene.manchester.ac.uk/learn/discovery-of-graphene/
Fischbein, M. D., & Drndić, M. (2008). Electron beam nanosculpting of suspended graphene sheets. Applied Physics Letters, 93(11). https://doi.org/10.1063/1.2980518/335402
Graphene Sheet Uses - Nanografi Nano Technology. (n.d.). Retrieved April 19, 2024, from https://nanografi.com/blog/graphene-sheet-uses/
Jiang, Z., Jiang, Z. J., Tian, X., & Chen, W. (2013). Amine-functionalized holey graphene as a highly active metal-free catalyst for the oxygen reduction reaction. Journal of Materials Chemistry A, 2(2), 441–450. https://doi.org/10.1039/C3TA13832A
Liu, T., Zhang, L., Cheng, B., Hu, X., & Yu, J. (2020). Holey Graphene for Electrochemical Energy Storage. Cell Reports Physical Science, 1(10), 100215. https://doi.org/10.1016/J.XCRP.2020.100215
Lokhande, A. C., Qattan, I. A., Lokhande, C. D., & Patole, S. P. (2020). Holey graphene: an emerging versatile material. Journal of Materials Chemistry A, 8(3), 918–977. https://doi.org/10.1039/C9TA10667G
Nanomaterials - Wikipedia. (n.d.). Retrieved April 19, 2024, from https://en.wikipedia.org/wiki/Nanomaterials
Nanotechnologies: 3. What are the physical and chemical properties of nanoparticles? (n.d.). Retrieved April 19, 2024, from https://ec.europa.eu/health/scientific_committees/opinions_layman/en/nanotechnologies/l-2/3-nanoparticle-properties.htm
Rajput, N. S., Al Zadjali, S., Gutierrez, M., Esawi, A. M. K., & Al Teneiji, M. (2021). Synthesis of holey graphene for advanced nanotechnological applications. RSC Advances, 11(44), 27381–27405. https://doi.org/10.1039/D1RA05157A
Top-Down Nanoparticle Synthesis - Nanografi Nano Technology. (n.d.). Retrieved April 19, 2024, from https://nanografi.com/blog/topdown-nanoparticle-synthesis/
Recent Posts
-
Nanocomposites in Food Packaging
The utilization of nanocomposites in food packaging represents a significant advancement in the fiel …19th Apr 2024 -
What is the Difference Between 7075 and 6061 Aluminum Alloy?
When comparing 7075 aluminum alloy to 6061 aluminum alloy, it's essential to understand their disti …5th Apr 2024 -
Iron-Air Batteries: The Ultimate Guide
Iron-air batteries represent a significant breakthrough in energy storage technology, offering a sus …29th Mar 2024